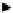Environmental Engineering Reference
In-Depth Information
Thermochemical conversion
Entrained-flow gasification
(EFG)
Pyrolysis
o
Clean and
environmentally
friendly energy
carrier
o
Chemicals and
electronic devices
production
o
Hydrogenated fats
and oils in food
industry
o
Steel processing
and desulfurization
o
Reformulation of
gasoline in
refineries.
Indirect/CFB
gasification
Biomass
Steam
reforming/
partial
oxidation
o
Forests
o
Agriculture
o
Wa ste
Supercritical water
gasification
Anaerobic digestion
Dark + photo fermentation
Direct / indirect bio-photolysis
Water
Biochemical conversion
Fig. 8.3
Renewable Hydrogen energy system
8.3.2
Dark Fermentation to Produce Hydrogen
Dark hydrogen fermentation processes produce gases which mainly contain hydro-
gen and carbon dioxide but in addition may also contain methane, carbon monoxide
and hydrogen sulfide, depending on the system and substrate. The basic building
block of cellulose (glucose) could in theory produce up to 12 mol hydrogen per
mole of glucose if completely oxidized to hydrogen and carbon dioxide (Eq. 8.1).
However, there would be no metabolic energy in this case and this has not been
reported.
C H
O
+ → +
6H O
12H
6CO
∆ =+
G
3.2 kJ
(8.1)
6
12
6
2
2
2
0
Hydrogen is mainly produced along with acetate and butyrate production pathways,
as indicated by Eqs. 8.2 and 8.3, with a greater H
2
yield when coupled with acetate
rather than butyrate.
C H
O
+ → +
2H O
4H
2CH COOH
+
2CO
∆ =−
G
206kJ
(8.2)
6
12
6
2
2
3
2
0
C H
O
→+
2H
CH CH CH COOH
6
12
6
2
3
2
2
+
2CO
∆ =−
G
254k
J
(8.3)
2
0
Dark fermentation thus offers the potential to produce 3-4 mol H
2
/mol glucose.
The use of mixed cultures for dark fermentation offers more practical advantages