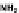Biology Reference
In-Depth Information
cisplatin in cancer cell lines.
139
Another example is the
overexpression of TdT in certain forms of leukemia
that also causes resistance to DNA damaging agents
that create DSBs.
140
through the generation of single- and double-stranded
DNA breaks.
A potential pitfall in broadly applying this strategy to
all DNA damaging agents lies primarily within the
redundancy in DNA pathways for certain DNA lesions.
As described earlier, there are at least four major path-
ways that can repair damage caused by alkylating
agents. These include direct repair of the alkylated base
by O
6
-alkylguanine-DNA methyltransferase
143
and indi-
rect repair through NER,
144
BER,
145
and recombination
pathways.
146
These last three pathways are complex
processes that require an ensemble of proteins to recog-
nize the damaged DNA, excise the modified region,
and then accurately re-synthesize the processed DNA.
The redundancy in the activity of these repair pathways
can lead to the effective removal of the lesion and thus
limit the utility of a compound. In addition, drug resis-
tance can occur if any of these pathways are up regu-
lated. However, one commonality that emerges
amongst all repair pathways is the absolute requirement
for DNA polymerase activity to re-synthesize the
repaired genomic material. As a consequence, selectively
inhibiting the polymerases involved in the repair process
should also sensitize cells to the cytotoxic effects of
various DNA damaging agents.
The major therapeutic strategy used to inhibit DNA
polymerases is to take advantage of its high efficiency
GENERAL STRATEGIES TO INHIBIT DNA
POLYMERASE ACTIVITY
Most strategies to enhance the therapeutic activity of
DNA damaging agents have focused on inhibiting the
enzymes involved in the recognition and/or excision
of DNA lesions. In general, inhibiting DNA repair
enzymes sensitizes cells to the cytotoxic effects of DNA
damaging agents. In a clinical setting, this is predicted
to generate favorable pharmacodynamic and pharmaco-
kinetic effects as lower drug concentrations will be
needed to induce cell death. This approach was first
demonstrated using O
6
-benzylguanine to increase the
efficacy of DNA damaging agents such as BCNU and cy-
clophosphamide.
141,142
O
6
-benzylguanine potently inacti-
vates O
6
-alkylguanine-DNA alkyltransferase,
141
and
this inhibition overwhelms the ability of the base exci-
sion and nucleotide excision repair pathways to
completely repair the lesions caused by BCNUand cyclo-
phosphamide.
142
This ultimately leads to apoptosis
dATP
A
T GCA G G T
dNTPs
Processive DNA synthesis
and cell
s
urvival
A
C G T C C
A
GCA G G T
T GCA G G T
F-ara-ATP
dNTPs
FA
T GCA G G T
Termination of DNA synthesis
and induction of apoptosis
FIGURE 5.9
“Trojan Horse” strategy of using nucleoside analogs to inhibit DNA polymerization. The polymerase is provided with
a modified nucleotide in which the 3'-OH group required for DNA elongation is missing, replaced with a halogen, or altered in configuration
from a normal ribose sugar. Since the nucleobase component is left unmodified, the polymerase incorporates the nucleotide analog into DNA as
efficiently as its natural counterpart. After incorporation, the nucleotide lacking a usable 3'-OH group is refractory to elongation causing the
induction of apoptosis by the termination of DNA synthesis.