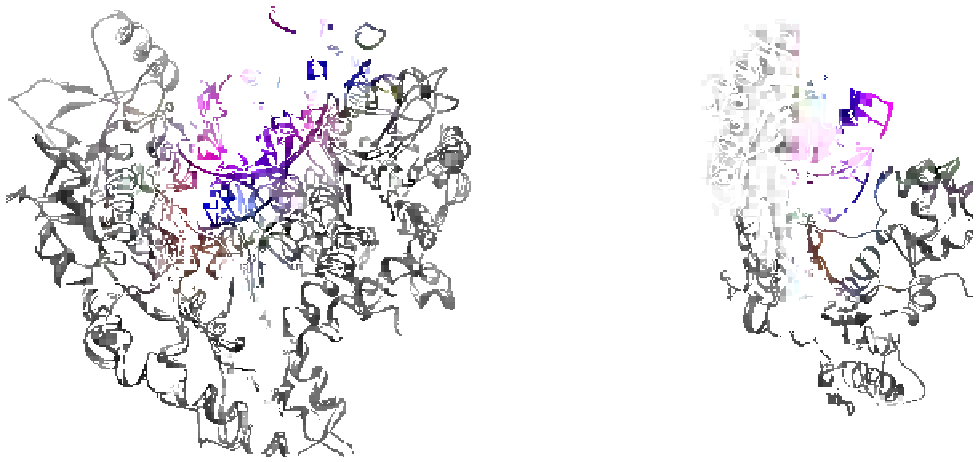Biology Reference
In-Depth Information
opposite bulky adducts such as N
2
-acetylaminofluorene-
G lesions
64
and N
2
-benzo(a)pyrene diolepoxide-G
lesions.
65
In addition, pol
k
catalyzes extension beyond
aberrant primer-terminal base pairs resulting from the
incorporation of nucleotides opposite DNA lesions by
other specialized DNA polymerases such as pol
i
.
66
Pol
z
is similar as this polymerase works in concert with other
specialized DNA polymerases to perform efficient trans-
lesion DNA synthesis.
67
In many cases, pol
z
activity is
essential for extension beyond DNA lesions that have
been initially replicated by specialized DNA polymerases
such as pol
h
.
Specialized polymerases DNA such as pol
q
,
4
, and
s
have not been as extensively characterized as the
others, and thus their biological functions have yet to
be unambiguously determined. Human pol
q
is a proof-
reading-deficient polymerase that plays multiple roles
in translesion DNA synthesis and in somatic hypermu-
tation of immunoglobulin genes.
68,69
Pol
q
can replicate
beyond DNA lesions such as abasic sites and thymine
glycol, and may also be involved in the BER pathways
as it possesses 5'-deoxyribose phosphate (5'-dRP) lyase
activity that is used during single-nucleotide BER.
70
Although mice defective in pol
q
are viable, they have
elevated spontaneous and radiation-induced frequen-
cies of micronuclei in circulating red blood cells sug-
gesting that this polymerase is also involved in
processing DNA damage that leads to the formation
of DSBs.
71
Structural Features of DNA Polymerases
The amino acid sequences of the DNA polymerase
proteins have been deduced from the genetic code of
numerous organisms including viruses, bacteria,
and eukaryotes.
72,73
Analysis of these primary amino
acid sequences reveals some very important similarities
in specific domains of these enzymes, and these similar-
ities indicate significant conservation of DNA polymer-
ases throughout evolution. In general, only a few amino
acids are conserved amongst all DNA polymerases, and
these include carboxylic acid containing amino acids
that are important for catalysis during DNA polymeri-
zation by binding metal ions and/or activating the
3'-OH of the primer for catalysis. However, despite
low overall primary amino acid sequence identity,
all DNA polymerases characterized to date share
several common structural features. In general, the
overall structure of DNA polymerases resembles a
“right hand” containing fingers, palm, and thumb
subdomains (reviewed elsewhere
75
e
77
)(
Figure 5.3
). Of
these domains, the palm is the most closely conserved
structural feature as it contains the conserved carboxylic
acids that function to coordinate metal ions that are
needed for the chemistry of phosphoryl transfer. The
fingers domain interacts with the incoming dNTP as
well as the templating base and thus plays an important
role in nucleotide selection. The thumb domain plays
dual roles by positioning duplex DNA for the incoming
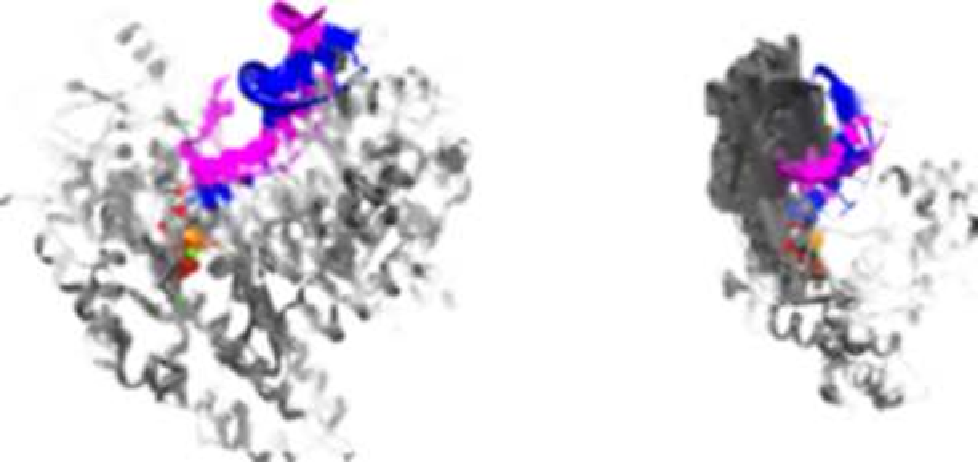
RB69 DNA polymerase
“Classical DNA polymerase”
Pol
η
“Non-classical DNA polymerase”
Duplex
DNA
Duplex
DNA
Fingers
Thumb
Thumb
Fingers
Palm
Palm
FIGURE 5.3
X-ray crystallographic structures of high-fidelity replicative DNA polymerase (left panel) and low-fidelity “specialized” DNA
polymerase (right panel). (
Please refer to color plate section
).