Biology Reference
In-Depth Information
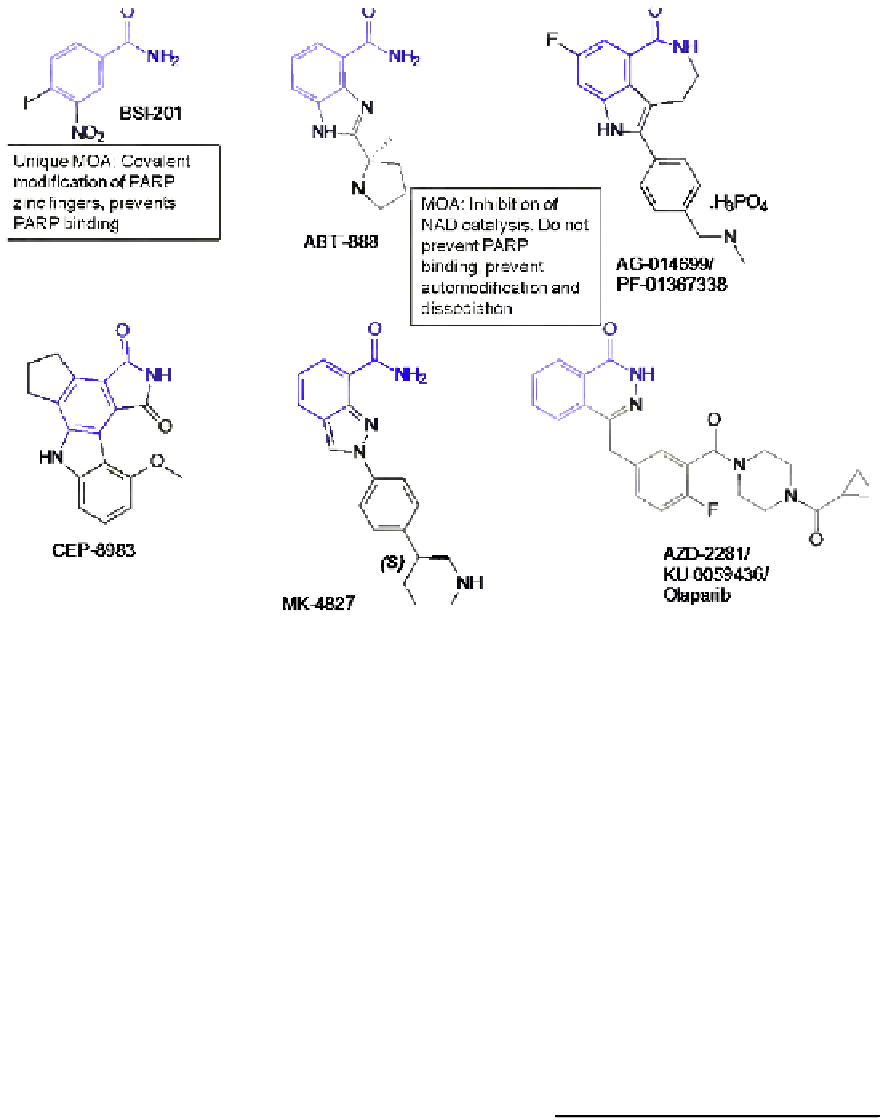
FIGURE 4.2
Chemical structures of PARP inhibitors in current clinical development.
break repair and increased cell death following exposure
of L1210 cells to the DNA methylating agent, DMS, and
it was first proposed that PARP inhibitors might be
useful in chemotherapy.
50
Subsequently 3AB was also
shown to enhance the activity of ionizing radiation.
51
Various academic and industrial groups began further
PARP inhibitor development based on the nicotinamide/
benzamide pharmacophore and both structure
and therefore do not inhibit PARP-1 binding to DNA
but do inhibit its polymer formation and promotion of
DNA repair; indeed they may further hamper repair by
physically obstructing the access of repair proteins. An
exception to this mode of action is 4-iodo-3-nitrobenza-
mide, which, although containing the nicotinamide phar-
macophore, is proposed to covalently modify PARP-1
and prevent PARP-1 binding to DNA through ejection
of the zinc from the zinc fingers.
58
This inhibitor also
interferes with glycolysis through inhibition of GAPDH
and should therefore not be considered to be representa-
tive of PARP inhibitors as a whole.
59
activity
relationship studies
52
e
55
and “analogue by catalogue”
studies
56
revealed that the orientation of the carboxamide
group in the “anti” conformation with respect to the ben-
zamide ring was critical for potency and that constrain-
ing it in this orientation by incorporation into a ring, or
through intramolecular hydrogen bonding increased
the potency. Co-crystalization of these early inhibitors
within the PARP catalytic domain confirmed that this
orientation of the carboxamide was essential for
hydrogen bond interactions with critical amino acid resi-
dues in the active site
16,57
such that the carboxamide
oxygen forms two hydrogen bonds with Ser904-OG
and the Gly863-N, and the adjacent amide nitrogen on
the inhibitor donates a hydrogen bond to Gly863-O.
Because of the structural similarity between PARP-1
and PARP-2 the inhibitors show little, if any, selectivity
between these two enzymes. This can be considered an
advantage in that both DNA damage-activated enzymes
are inhibited. These PARP inhibitors are, by and large,
catalytic inhibitors competitive with respect to NAD
þ
e
CHEMOPOTENTIATION
AND RADIOPOTENTIATION
IN VITRO
AND
IN VIVO
There is abundant
in vitro
and
in vivo
evidence
demonstrating a role for PARP-1/2 in the repair of
DNA damage and cell survival following exposure to
DNA methylating agents, topoisomerase I poisons and
ionising radiation and radiomimetics obtained using
the more potent and selective inhibitors. These studies
have been confirmed by genetic inactivation of PARP-1
and PARP-2. There are numerous reviews on these
inhibitors
60
e
68
and this chapter will deal only with the
major biologically significant observations.