Biology Reference
In-Depth Information
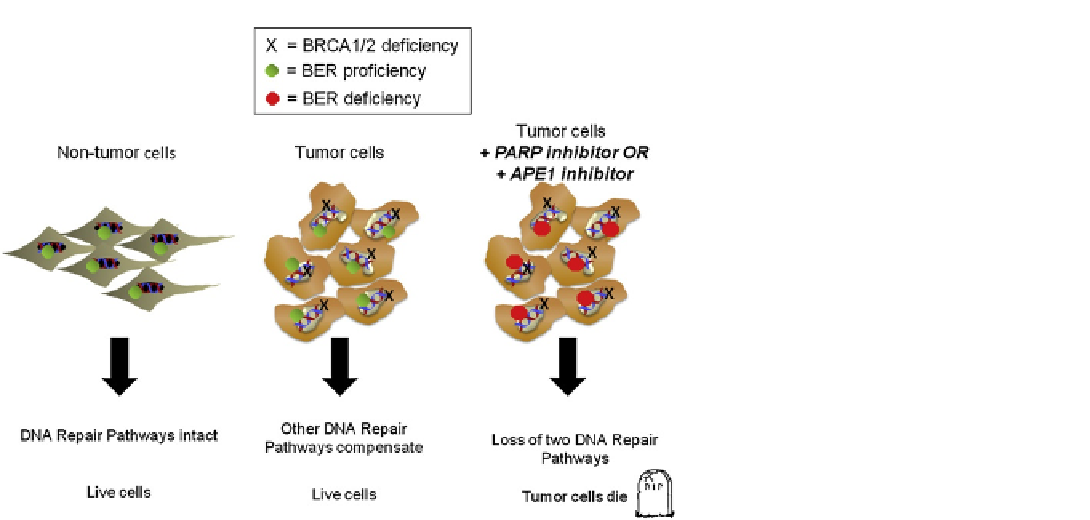
FIGURE 3.5
Differential effects of BER inhibi-
tors in normal and tumor cells based on their
BRCA (breast cancer type I susceptibility protein)
status. The non-tumor cells would have functional
BER and two copies of BRCA proteins and be
competent to repair their DNA. Tumor cells have
acquired a mutation in BRCA proteins which
diminishes their DNA repair capacity but the other
DNA repair pathways are able to compensate and
the tumor survives. When these BRCA-deficient
tumor cells are challenged with a BER inhibitor
their capacity for DNA repair is severly compro-
mised and they are unable to survive. This idea
that it takes two hits to kill the tumor cell is
referred to as synthetic lethality. Clinical trials in
a patient population enriched for BRCA deficiency
with PARP inhibitors are showing clincal success.
(
Please refer to color plate section
).
are showing clinical utility and solidifying claims that
DNA repair inhibitors have a place in the treatment of
cancer.
187,260,264
(For more detail about PARP inhibition,
please see Chapter 4).
The first studies of PARP inhibitors in humans were
conducted in patients with advanced solid tumors and
enriched for patients with BRCA mutations, especially
ovarian and breast. In a phase I trial, PARP inhibitor ola-
parib (AZD2281, KU-0059436) was not overly toxic,
demonstrating a similar or somewhat better toxicity
profile to most chemotherapeutics. This first trial
demonstrated that PARP inhibitors were safe, had
improved efficacy, but not enhanced toxicity, in the
cohorts of patients that were BRCA1/2 deficient. This
trial provides evidence that the idea of synthetic
lethality is critical in the tumors' response to PARP
inhibitors. Other PARP inhibitors that are under investi-
gation as single agents in advanced solid tumors (with
an emphasis on patients with the BRCA mutation) are:
Iniparib (BSI-201), MK4287, Valiparib (ABT888).
As discussed with APE1 inhibitors, combination
therapy using agents that generate DNA damage in
which PARP activation would be critical is a reasonable
approach. In preclinical models, PARP inhibitors have
demonstrated synergy with alkylating agents, platinat-
ing agents, topoI poisons and IR in a variety of tumor
cell lines and animal models. Consistent with the idea
of synthetic lethality, many groups investigated the
impact of other DNA repair pathways on sensitivity
to PARP inhibitors in cancer cell
but p53 status did not seem to affect the efficacy of
PARP inhibition.
264
Several trials are now under investi-
gation to determine if PARP inhibitors can sensitize
tumors to chemotherapeutic agents such as TMZ, carbo-
platin, gemcitabine, topotecan, paclitaxel, and cyclo-
phosphamide.
2,260
A wide variety of tumor types are
included in these clinical trials including breast, ovarian,
glioma, melanoma, and lung. One noteworthy study
demonstrating the utility of PARP inhibitors in combina-
tion chemotherapy regimens is in a Phase 2 trial with
triple-negative breast cancer (estrogen receptor-, proges-
terone receptor- and HER2-negative).
265
This trial evalu-
ated the addition of PARP inhibitor BSI-201 to
a gemcitabine/carboplatin regimen. The Overall
Response Rate (ORR) was significantly increased to
52.5% (from 32.3%) with the addition of BSI-201, and
the overall survival (OS) was increased by almost 5
months. In this study, the addition of PARP inhibitor
to the regimen did not increase the myelosuppressive
effects of the chemotherapy which is in contrast to other
combination studies. Dose limiting toxicity is one of the
potential concerns in the development of BER inhibitors:
increases in toxicity to the patient especially in combina-
tion with other dose-intense agents. Some potential solu-
tions or alternatives are careful scheduling of DNA
repair inhibitors and pairing them with radiation due
to the ability and precision of locally treating with
radiation.
2
In conclusion, despite some concern over the conse-
quences of inhibiting DNA repair as a cancer treatment,
initial trials with PARP inhibitors are showing promise
and suggesting that tumors may be sensitized to DNA
repair inhibitors selectively over normal cells. Several
lines. The activity
of DNA repair pathways
can
affect the synergy of alkylating agents, radiation, and
topoI poisons in combination with PARP inhibitors,
BER, MMR, and HR
e
e