Biology Reference
In-Depth Information
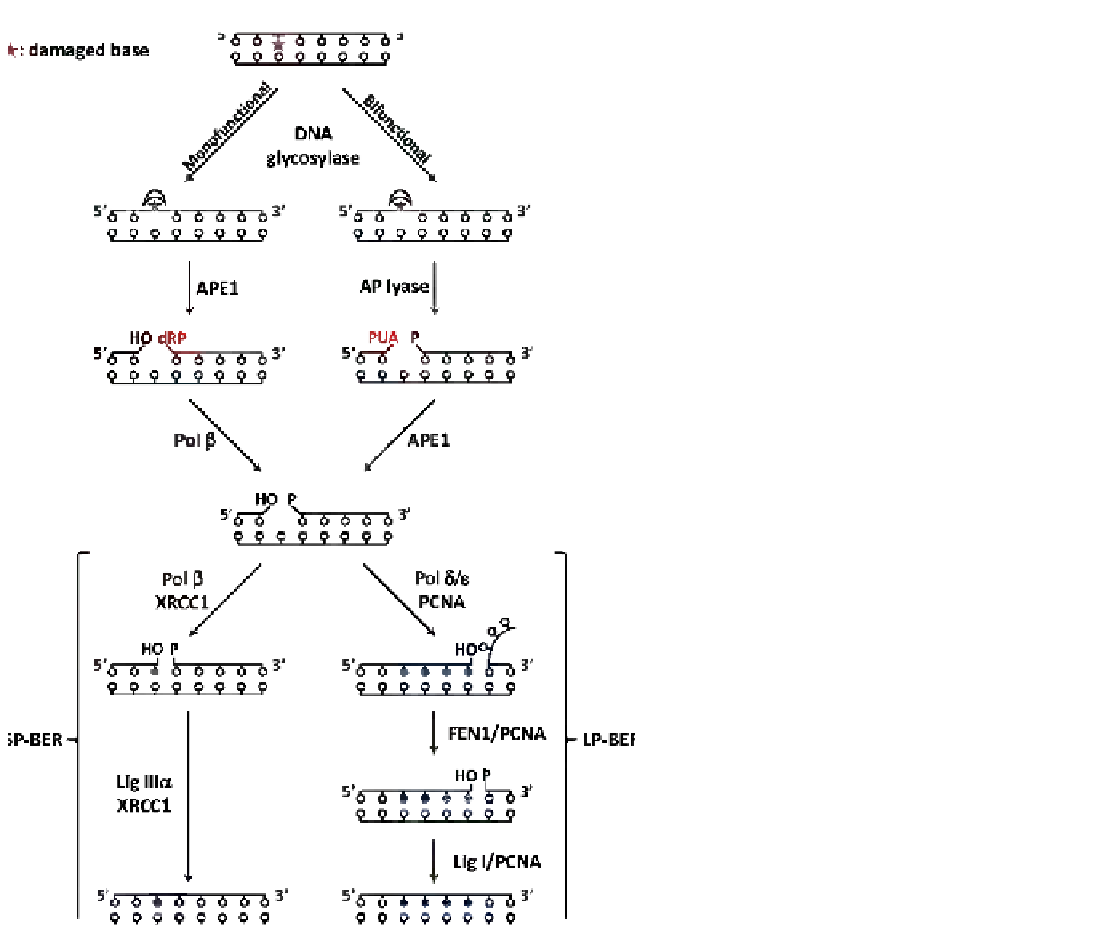
FIGURE 3.2
Schematic illustration of the BER
pathway. The damaged base is represented by a star.
DNA glycosylases initiate BER by excising damaged
bases from DNA and generating an abasic site. If the
pathway is initiated by a monofunctional DNA glyco-
sylase, APE1 hydrolyzes the phosphate bond at 5' to the
AP site leaving a 3'-OH group and a 5'-dRP termini
flanking the nucleotide gap. Then, Pol
b
excises the 5'-
dRP moiety generating a 5'-P. If the pathway is initiated
by a bifunctional DNA glycosylase, after excising the
base the AP lyase hydrolyzes the 3' bond to the AP site
leaving a phospho
a
,
b
-unsaturated aldehyde (PUA)
abasic fragment. APE1 processes the 3' termini gener-
ating a 3'-OH group. At this point BER can proceed
through the short-patch (SP-BER) where Pol
b
intro-
duces a single nucleotide past the abasic site and Lig
III
a
seals the DNA nick. On the contrary, in the long-
patch (LP-BER) Pol
d
/
3
introduces two to eight nucle-
otides past the abasic site. The resulting overhang DNA
is excised by FEN1 endonuclease and the nick sealed by
DNA ligase I. In addition to the BER enzymes, many of
the associated scaffold proteins that are reported in the
text are also shown.
sugar.
34
Then, the AP lyase activity eliminates the phos-
phate group 3' of the nucleotide lesion. The remaining 3
0
phospho-
a
,
b
-unsaturated aldehyde (PUA) abasic frag-
ment is a substrate of AP endonucleases, and their action
leads to a single-nucleotide gap that will be filled by
DNA polymerases.
35
In recent years, high-resolution
structures of a number of DNA glycosylases have been
obtained, providing insight into how these enzymes
overcome the significant challenge of specifically recog-
nizing small base modifications in the presence of vast
excess of unmodified bases.
7
Despite differences in the
folds and specific residues used to recognize damaged
bases, unifying common themes for BER initiation
have emerged. Among these, extrahelical flipping of
the damaged base into a lesion-specific recognition
pocket is particularly intriguing, as it must rely on an
intrinsic property of the damaged DNA. All DNA glyco-
sylases studied to date bind to the minor groove, kink
DNA at the site of damage, and flip the lesion base out
of the DNA major groove. Thus, an initial step in recog-
nition evidently exploits the deformability of the DNA
at a base pair destabilized by the presence of a lesion.
Each glycosylase is necessarily damage-specific, so
only bases that can be accommodated in a defined
binding pocket upon nucleotide flipping provide the
necessary contacts and orientation for base excision.
25
The critical importance of the extrahelical base binding
pocket for glycosylase specificity was elegantly shown
first by Krokan and colleagues, who demonstrated that
the uracil pocket in uracil DNA glycosylase (UNG)
could be mutated to allow the removal of normal cyto-
sine and thymine bases
from DNA.
36
A second