Biology Reference
In-Depth Information
is upregulated in CHO cells and HeLa cells.
126
CREB is
under redox control for its binding to DNA and acti-
vating transcription. Therefore, there is a tight link and
interaction in the DNA BER process between a number
of transcription factors that are under redox control
and the DNA repair response (
Figure 11.8
).
Nucleotide excision repair (NER) is divided into tran-
scription coupled repair (TCR) and global genome
repair (GGR), and is affected differentially by p53.
Several studies found p53 selectively affected GGR,
but not TCR. Two main proteins in GGR, DDB2 and
XPC, are involved in DNA damage recognition and
are transcriptionally regulated by p53.
127
e
129
Genomic
instability results from loss of p53 and subsequent defi-
ciencies in the GGR proteins DDB2 and XPC as demon-
strated in knockout mouse studies. In this study, 100% of
XPC
-/-
mice develop lung cancer, and DDB2
-/-
mice
develop skin tumors.
130
Thus, redox regulation of p53
directly affects expression of DDB2 and XPC, which
are required for GGR (
Figure 11.8
).
Mismatch repair (MMR) removes mismatches in
DNA introduced by DNA polymerases. MSH2 in
complex with MSH6 recognizes single base mismatches
and short insertion/deletion mispairs, while MSH2 in
complex with MSH3 recognizes larger loops of unpaired
nucleotides. MSH2, MLH1, and PMS2 have all been
shown to be regulated by p53 similar
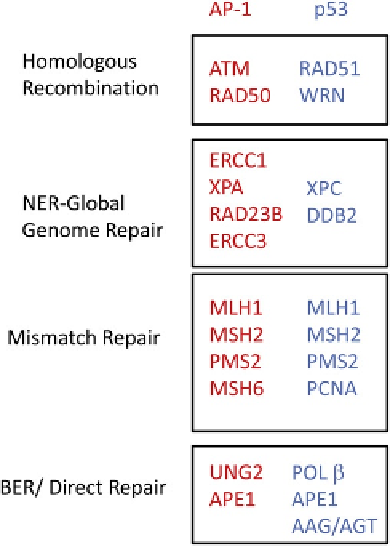
FIGURE 11.8
Both AP-1 and p53 induce expression of a number
of DNA repair genes involved in homologous recombination, nucle-
otide excision repair/global genome repair, mismatch repair, base
excision repair, and direct repair (adapted from Luo et al.
9
). The genes
induced by each transcription factors are shown within each DNA
repair pathway.
to DDB2,
XPC
131,132
and PCNA
133
in NER.
Double-strand breaks (DSBs) threaten severely
genomic stability by facilitating deletion and/or trans-
location of chromosomal DNA. Homologous recombi-
nation (HR) may lead to genomic instability due either
to a deficit or an excess of this repair mechanism, and
is therefore highly regulated. The tumor suppressor
protein p53 plays an important role in the repair of
DSBs through the regulation of both DSB repair path-
ways, HR and non-homologous end joining (NHEJ).
Mice deficient in wild-type p53 show increased levels
of HR.
134,135
The mechanism by which p53 inhibits
HR is through repression of RAD51 expression.
136
Other proteins repressed through transcriptional
mechanisms by p53 include RecQ4 helicases, WRN
and RecQ4.
137,138
Finally, an enzyme involved in direct repair, AGT, is
also transcriptionally regulated. In murine fibroblast
cell lines, AGT has been shown to be regulated by
p53
139,140
and also by NF-
k
B, another transcription
factor that is under redox control by APE1. This was
demonstrated by overexpression of the p65 subunit of
NF-
k
B in HEK293 cells resulting in an increase in AGT
expression.
141
These studies point to the relevance of redox control
in DNA repair responses through p53. Therefore, if
reduced p53 is required to bind DNA and either activate
or repress the transcription of DNA repair genes as
response to
g
-ray treatment, p53 promoted activation of
AAG. However, this initial step in BER was also shown
to be under negative transcriptional regulation by p53
after exposure to nitric oxide.
124
Recent studies have
demonstrated that p53 downregulated APE1 expression
through binding to the promoter region of APE1 that
includes an SP1 site. APE1 mRNA and protein levels
decreased in a time-dependent manner in the human
colorectal cancer line HCT116 p53(
), but not in the
isogenic p53 null mutant after treatment with campto-
thecin. Overexpression of wild-type p53 in the p53 null
cells significantly reduced both endogenous APE1 and
APE1 promoter-dependent luciferase expression in
a dose-dependent fashion.
125
Thus, Ref-1 (APE1) regu-
lates DNA-binding of p53, and p53 in turn regulates
both expression and protein levels of APE1 in this
example.
Another BER enzyme that is regulated by p53 is DNA
polymerase
b
(pol
b
), which is involved in short patch
BER. Pol
b
has associated deoxyribose phosphate lyase
activity that is important and often rate-limiting in
BER and acts following APE1 activity. Pol
b
also plays
a role in single nucleotide gap filling in long-patch
BER. Expression of pol
b
protein expression is altered
in p53-deficient cells.
88
Additionally, in response to
DNA alkylating agent exposure, pol
b
gene expression
þ
/
þ