Biology Reference
In-Depth Information
First a global QTOF mass spectrometric analysis was
used to establish whether APE1 forms a covalent adduct
with E3330.
102
Under both native and denaturing nano-
spray conditions, there was little evidence of formation
of a covalent adduct with E3330. This was a somewhat
surprising result given the SPR data reported for the
interaction of E3330 with APE1 with a K
D
of 1.6
10
9
M.
43
However, the APE1 used for this study was
purified under denaturing conditions and then refolded
with no data shown for endonuclease activity for the
refolded sample. In this same report, APE1 was purified
from nuclear Jurkat cell extracts using E3330 attached to
beads firmly establishing a direct interaction between
APE1 and E3330.
43
One possibility is that E3330 revers-
ibly modifies APE1.
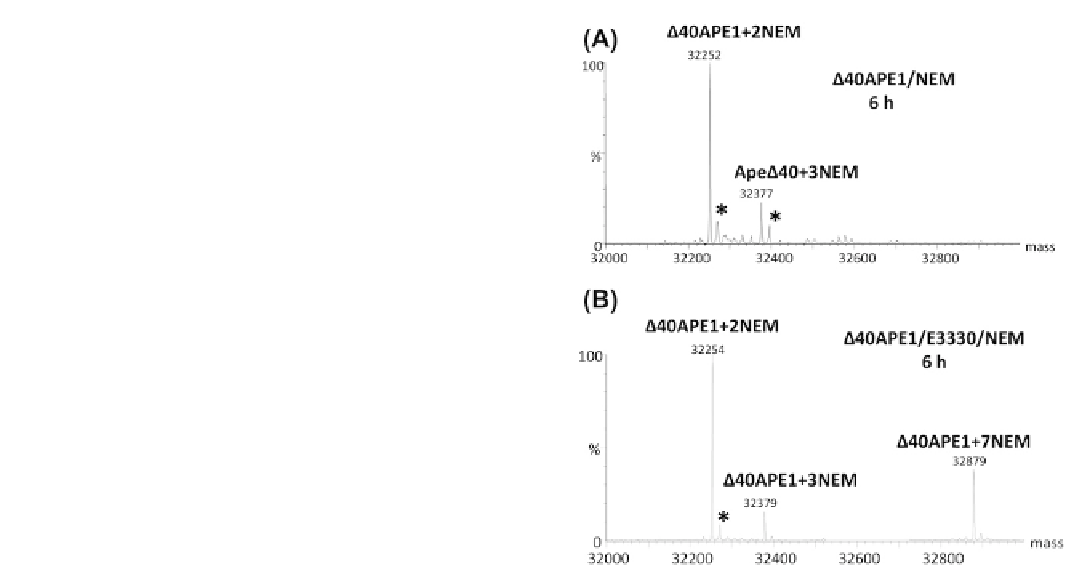
To further investigate the nature of the interaction
between E3330 and APE1, a chemical footprinting assay
was developed using NEM to report on the accessibility
of Cys residues within APE1.
102
In the absence of E3330,
modification of APE1 with NEM results in formation of
a
2 NEM product in 100% yield for a 30 min reaction at
room temperature. Incubation of APE1 with NEM and
E3330 over the course of several hours at room temper-
ature resulted in the formation of two major products,
þ
þ
FIGURE 11.7
ESI mass spectra of
D
40APE1 after incubation
without (A) and with E3330 (B) in the presence of NEM for 6 h.
Samples were incubated in 10 mM HEPES with 150 mM KCl at pH
7.5([protein]
2 NEM and
þ
7 NEM products (
Figure 11.7
). Small
500
m
M). The symbol *
denotes peaks for the water adducts. Mass spectra were collected on
a Waters Micromass Q-TOF instrument and deconvolution was done
with MaxEnt1 algorithm provided with that system. Adapted from Su
et al.
102
¼
100
m
M; [E3330]
¼
[NEM]
¼
amounts of a
3 NEM product were also observed.
LC-MS/MS analysis of the samples revealed that the
þ
þ
2 NEM product was modified on the solvent accessible
Cys residues 99 and 138, the
7 NEM product was
labeled on all Cys residues, and the
þ
3 NEM product
resulted from modification of other non-Cys residues
within the protein.
As the
þ
folded state. To test this idea, the experiments using
NEM as a chemical footprinting agent were repeated
at 37
C. At higher temperature, we observed a small
percentage of
2 NEM product formed first and was over
time converted to the
þ
7 NEM product with concomi-
tant decreases in the amount of
þ
7 NEM product even in the absence of
E3330 consistent with the ability of APE1 to adopt an
alternate conformation. In the presence of E3330 at
37
C, we observed a large increase in the percentage
of
þ
2 NEM product, it
was of interest to determine whether modification by
NEM altered the redox properties of the protein through
formation of the
þ
2 NEM.
102
Accordingly, APE1 was
modified with NEM, verified by global QTOF analysis,
and then assayed for redox activity. It was found to
retain ~90% of redox activity observed for unmodified
APE1. Another point of consideration was whether
E3330 was just acting as a denaturant in the assay. To
test this, APE1 was incubated with E3330 for 24 hours,
followed by reaction with NEM for 30 min, and then
analysis of product formation by global QTOF mass
spectrometry. Only the
þ
7 NEM product, now about 80% of the labeled
protein. These results are consistent with the idea that
APE1 adopts more than one conformation, particularly
at the physiologically relevant temperature of 37
C.
To determine whether specific Cys residues played
a role in allowing APE1 to adopt an alternate confor-
mation, several substituted APE1 samples were tested
in the NEM footprinting assay.
102
The role of the redox
critical Cys 65 was examined first. As before, the
þ
2
NEM product formed rapidly and was followed by
slow formation of a
þ
2 NEM product was observed;
thus, E3330 does not denature APE1. Based on these
results, we concluded that E3330 may recognize
a partially or locally unfolded state of APE1 that exists
in equilibrium with the fully folded form observed in
the crystal structures. Therefore, interaction with E3330
may shift the equilibrium between these states of
APE1. Modification by NEM traps the
þ
6 NEM product. Thus, Cys 65
is not required for APE1 to adopt an alternate confor-
mation. A second possibility was that modification of
both Cys 99 and Cys 138 by NEM somehow facilitated
the unfolding of APE1 resulting in the formation of
a
þ
7 NEM product. Therefore, C99A/C138A APE1
was tested in the NEM footprinting assay and found
to slowly form a
þ
7 NEM state
of the enzyme preventing it from returning to its fully
þ
þ
5 NEM product over time in the