Biology Reference
In-Depth Information
has found use in the treatment of hepatocellular carci-
noma in Japan.
36
Antibody-conjugated calicheamicin
derivatives have also been developed; gentuzumab ozo-
gamicin (Mylotarg
(A)
) has been approved by the US FDA
for treatment of refractory acute myeloid leukemia,
37
and inotuzamab ozogamicin is in clinical trials for the
treatment of non-Hodgkin's lymphoma.
38
(B)
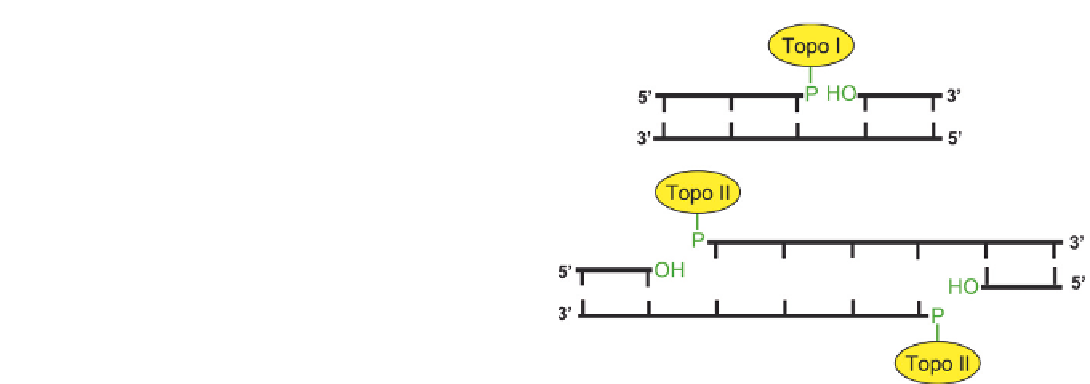
Topoisomerase Inhibition
Several enzymes involved in DNA metabolism
cleave either one or both DNA strands. These include
type I and II topoisomerases, which catalyze topological
changes in DNA that are essential for many processes
involving DNA including DNA replication, transcrip-
tion, recombination and repair. Mammalian topoiso-
merase I transiently cleaves one strand of the DNA
duplex by forming a covalent 3
0
-phosphotyrosyl inter-
mediate. The other strand break terminus has a 5
0
-
hydroxyl end group. The DNA then swivels about the
intact strand to relieve torsional strain and relax a super-
coil. The cleaved strand is then rejoined by the
enzyme.
39,40
Naturally occurring DNA lesions, such as
abasic sites, 8-oxoguanines, mismatches and DNA
single-strand breaks, within the cleavage recognition
sequence can influence topoisomerase I activity.
41,42
Importantly, abasic sites and single-strand breaks adja-
cent to the cleavage site can trap the cleavage
complexes.
41,43
When such complexes encounter DNA
replication forks, the SSBs can be converted to DSBs.
The repair and ligation of such breaks will be impeded
both by the 3
0
-phosphotyrosyl bond to the topoisomer-
ase and by the 5
0
-hydroxyl terminus (
Figure 8.4A
).
Topoisomerase II catalyzes the transient cleavage of
both strands of duplex DNA and the passage of another
DNA duplex through the protein-linked “gate”.
40,44
The
scissile bonds on the opposite strands are four base pairs
apart. In this case cleavage of the DNA is accomplished
via the formation of covalent 5
0
-phosphotyrosyl-enzyme
bonds on each cleaved DNA strand (
Figure 8.4B
). After
passage of the second duplex, the broken strands
undergo ligation. As with topoisomerase I, DNA lesions
close to the scissile bonds can alter topoisomerase II
activity. In particular, intact and cleaved abasic sites
can block religation and thus give rise to permanent
DSBs.
45,46
Both type I and type II topoisomerases have been
chemotherapeutic targets for many years.
47,48
In the
case of topoisomerase I, one of the early inhibitors iden-
tified was a plant alkaloid, camptothecin, and two
water-soluble camptothecin derivatives are now widely
used in the clinic. Irinotecan is used to treat colorectal
cancer in both first- and second line-therapy.
49,50
As a
first-line agent, it is often combined with 5-fluorouracil.
It has also shown clinical activity against lung cancer.
51
FIGURE 8.4
Strand breaks induced by DNA topoisomerases.
(A) Topoisomerase I cleaves a single strand by forming a covalent bond
between a tyrosine and the DNA 3
0
-phosphate group. This cleavage
complex can be trapped due to the presence of adjacent DNA lesions,
such as abasic sites, or chemotherapeutic agents, such as irinotecan.
Repair of the trapped complexes requires cleavage of the phospho-
tyrosyl bond by tyrosyl-DNA phosphodiesterase (Tdp1), followed by
processing of the resulting 3
0
-phosphate and 5
0
-hydroxyl termini by
polynucleotide kinase (PNKP) before strand rejoining can occur.
(B) Topoisomerase II cleaves both strands at sites four base pairs apart
by forming covalent 5
0
-phosphotyrosyl bonds. This cleavage complex
can be trapped by chemotherapeutic agents, such as etoposide.
Irinotecan is hydrolyzed by carboxylesterase in the
liver to its active metabolite, 7-ethyl-10-hydroxycampto-
thecin (SN-38).
52
Topotecan, which does not require
metabolism for its activity, is indicated as a second-line
therapy in the treatment of ovarian and small-cell lung
cancers.
53,54
These compounds generate persistent
SSBs by stabilizing the topoisomerase 1-DNA cleavage
complex.
Drugs that target topoisomerase II are included in
approximately half of all chemotherapy regimens.
46
Clinical inhibitors of topoisomerase II fall into two cate-
gories. The first, often referred to as topoisomerase
poisons, comprise intercalating and non-intercalating
compounds that trap the topoisomerase II-DNA
cleavage complex, generating DSBs. Included in this
group of drugs are doxorubicin, mitoxantrone and eto-
poside. However, an important clinical complication
identified with the use of topoisomerase II poisons,
such as etoposide, is secondary malignancy, which
may be due to the high probability of generating chro-
mosomal translocations.
55
The second category of
inhibitors, exemplified by bisdioxopiperazine ICRF-
187, inhibit topoisomerase II ATPase activity and trap
theenzymeonDNAasaclosedclamp.
56
Despite the
lack of clear indication that this class of inhibitors
generates DSBs, there is evidence to suggest that non-
homologous end joining (NHEJ, discussed in detail
below) may play a role in the cellular response to these
agents.
57,58