Biology Reference
In-Depth Information
is only revealed upon DNA damage induction, i.e.,
ex
vivo
treatment.
The Powell laboratory has expanded on this approach
and in a preliminary analysis identified 6/30 breast
cancers as lacking a RAD51/BRCA1 response (20%).
131
Furthermore, instead of the use of core biopsies, an
approach involving fine-needle biopsy aspirates was
developed. Breast cancer aspirates were made into
a cell suspension in ~1 ml of phosphate buffered saline,
ex-vivo
irradiated or sham treated, incubated for 4 hours,
and fixed on glass slides after processing the cell suspen-
sion in a cytospin. It was thought that the staining
process was generally easier compared to using intact
tumor tissues, with less permeability problems and
a better signal-to-noise ratio.
In order to determine whether this type of
ex vivo
assay
approach is also suitable for the study of drug-induced
DNA damage responses, the Willers laboratory has sub-
jected breast as well as NSCLC explants to cisplatin treat-
ment.
183,397
Four or 24 hours after a 1 hour treatment,
samples were snap frozen for later analysis. Immunoflu-
orescence analysis of
g
-H2AX foci demonstrated that
cisplatin diffused through the tissues and staining for
PCNA identified cells residing in S-phase. In a panel of
13 NSCLC tumors, three tumors (23%) lacked the ability
to induce RAD51 foci in response to cisplatin, consistent
with a functional HRR defect.
While this type of functional
ex vivo
foci assay repre-
sents a potentially powerful tool for the detection of pre-
existing and clinically relevant defects within the
complex HRR pathway, several technical challenges
remain, including: (1) potential intra-tumoral heteroge-
neity in foci responses, (2) low fraction of cells in
S-phase, compared to cell lines, necessitating co-staining
to detect replication-associated RAD51 foci, (3) need for
quantification and automation of foci scoring, and (4)
potential changes in hypoxia/reoxygenation upon
removal of the tumor tissue from the patient and incuba-
tion in the laboratory at 20% oxygen. Data from the Wil-
lers laboratory and by Vaira
et al.
398
indicate that
adequately cultured tumor explants are viable for at
least 24 hours and up to 5 days, offering a promising
avenue for assessing not only foci responses but also
surrogate endpoints of cell fate such as apoptosis or
senescence, thereby allowing pharmacodyamic profiling
of human tumors.
Other promising
ex vivo
approaches involve the trans-
plantation of tumor explants into the fat pad of mice
(Human-In-Mouse models)
399
or the generation of
primary tumor cultures or cell lines from patients. As
an example for the latter, Mukhopadhyay
et al.
estab-
lished 25 primary cultures from ascites fluid of patients
with advanced ovarian cancer.
400
Ex vivo
treatment with
a PARP inhibitor showed that only 34% of tumors were
able to mount a RAD51 foci response.
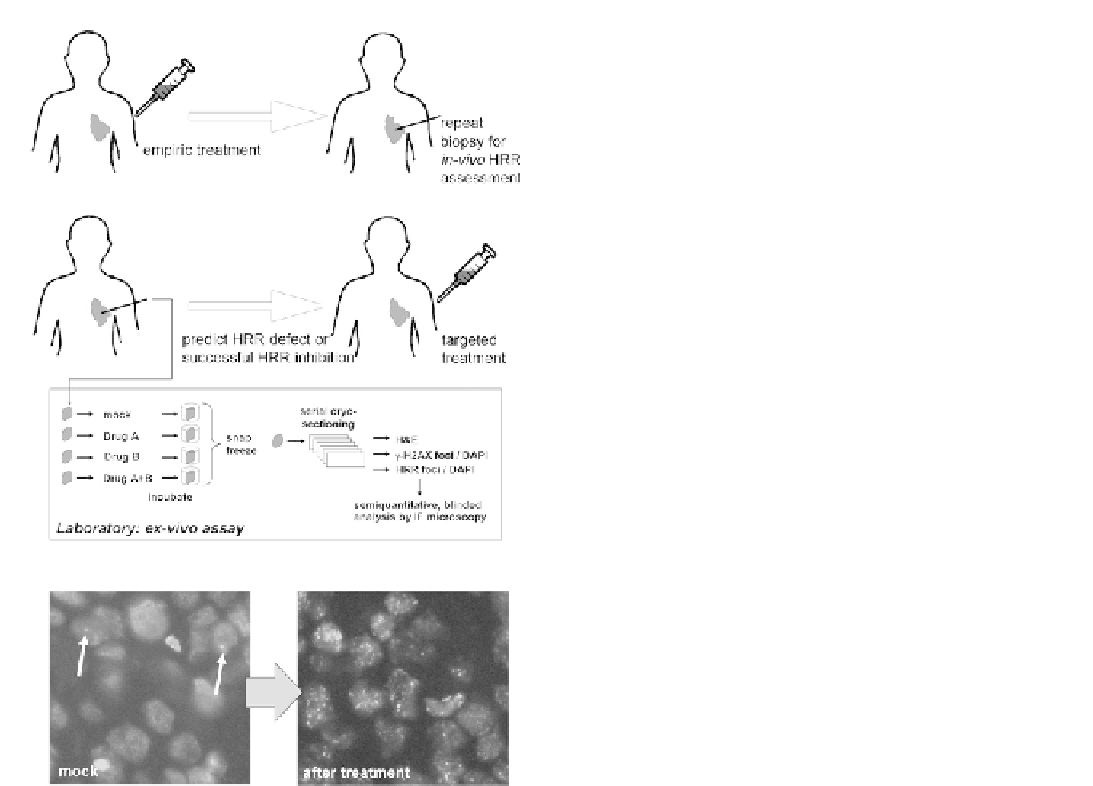
(A)
(B)
(C)
FIGURE 7.12
Foci assays in patients. In order to apply repair foci
assays in patients, it is necessary to measure foci response after the
induction of DNA damage. Because a rebiopsy of cancer patients after
initiation of treatment is often not feasible,
ex vivo
assays have been
developed in which live tumor biopsies or explants are subjected to
DNA damaging agents in the laboratory. See text for details.
treatment, samples were incubated in complete medium
in a cell culture incubator for 4 hours to allow for foci
formation and then snap-frozen in OCT for later anal-
ysis. Viable tumor was identified on serial 5
m
m sections
by H&E staining, and RAD51, BRCA1, and FANCD2
foci could be readily visualized in individual irradiated
cells. In three tumors, there was an intact foci response,
while the other four tumors lacked foci induction. Yet,
there was no difference in the baseline of foci numbers
in unirradiated tumor cells. Notably, three of the four
foci-defective tumors were triple-negative, a phenotype
associated with BRCA1 deficiency as reviewed above.
Yet, there was reduced BRCA1 expression by RT-PCR
in only two of the four specimens. Even though the
number of subjects in this study was small, these data
suggest that gene expression may indeed correlate
poorly with HRR pathway activity as measured by foci
formation, and further, that a functional pathway defect