Biology Reference
In-Depth Information
repair can be due to defects in the DDR. Thus, during
tumorigenesis there is assumed to be selection pressure
for defects in the DDR and indeed many different tumor
types harbor such defects in their DDR machinery.
124
Another threat to genomic integrity arises from acti-
vated oncogenes or loss of tumor suppressors. It has
been shown that activated oncogenes can lead to
increased proliferation, resulting in replication stress
and increased DNA damage such as DSB.
125
In precan-
cerous lesions, an increase in DSBs can be observed. If
there is a defect in the DDR resulting in attenuated cell
death signals, proliferation will continue despite the
DNA damage, leading to mutations, genomic instability
and ultimately to cancer development.
HRR and Carcinogenesis
HRR constitutes an ubiquitous, error-free DSB repair
pathway important for cell survival after exposure to
ionizing radiation and other DNA metabolism-based
anticancer agents. HRR also functions in the recovery
of stalled or collapsed replication forks as described
above. Thus, defective HRR will lead to error-prone
DSB repair and compromise proper replication of
DNA (reviewed in
126,127
). Conversely, hyperactive or
unscheduled HRR may lead to unwarranted genetic
exchanges.
128
HRR has evolved to be tightly regulated
to promote precise repair and limit genomic aberrations
and genetic loss. This is achieved through cell cycle
phase coordination, post-translational modifications,
and many accessory factors that either promote or
inhibit interactions.
126
Thus, for cancers, there exists
ample opportunity to deregulate this process.
How exactly selection pressure arises during the
process of carcinogenesis to disrupt HRR pathways is
currently unknown. However, given the crucial role of
HRR for the restart and repair of stalled replication forks
and the possibility for widespread genomic instability if
this process fails, it is tempting to speculate that replica-
tion-associated HRR is specifically targeted during
tumorigenesis. For example, one can envision that
increased levels of ROS with associated oxidative
damage may strain the ability of the HRR machinery
to restart stalled forks, and increased oncogenic
signaling could impair cell check point controls or
even HRR directly.
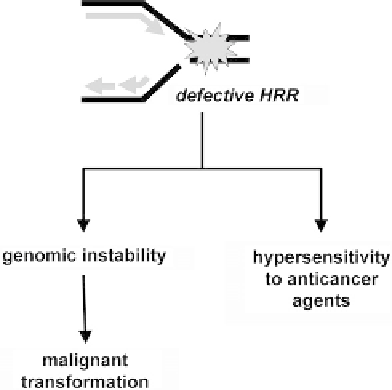
Clearly, a picture is emerging in which perturbations
of HRR in cancer cells are a more widespread cause of
genomic instability than previously appreciated.
129,130
Conversely, such cells may also be more sensitive to
certain chemotherapeutic agents and radiation. Thus,
the alterations in HRR that promote carcinogenesis by
causing genomic instability may also be the Achilles'
heel of the cancers that arise in this setting.
130
This
concept is illustrated in
Figure 7.4
.
FIGURE 7.4
Defective HRR as the “Achilles Heel” of cancer.
Human Cancer Predisposition Syndromes
with HRR Defects
Familial Breast Cancer
Hereditary breast cancer accounts for approximately
5
e
10% of breast cancer (reviewed in
1,131
). Mutations
in BRCA1 and BRCA2 account for the majority of fami-
lies with hereditary susceptibility to breast and ovarian
cancer. Women carrying a heterozygous germ line muta-
tion in one of these genes have an up to 80% lifetime risk
of developing breast cancer. In addition to the breast
cancer risk, women with BRCA1 mutations have an
increased risk of ovarian cancer. BRCA2 carriers are
also at an increased risk for developing ovarian cancer
as well as other solid tumors including prostatic and
colorectal adenocarcinoma, and additionally are at
a higher risk for male breast cancer.
While BRCA mutation carriers are heterozygous
within the germ line, malignant transformation requires
inactivation of the remaining wild-type allele in the
somatic target cells (reviewed in
131
). The sequence of
events which results in this mutation is not clear, but
there must be additional genetic or epigenetic changes,
such as p53 mutation, that must occur to allow these
HRR-deficient tumor cells to grow. Bi-allelic knock-out
of Brca1 or Brca2 is embryonic lethal in mice,
132,133
which represents a tumor suppressor paradox for these
proteins. The reason for the growth advantage associ-
ated with loss of BRCA1 or BRCA2 function is likely to
be connected to the genomic instability associated with
either the heterozygous state (haploinsufficiency) or
even the homozygous defective state, which allows
secondary mutational changes to result
in further
genomic instability and tumor progression.
A defect in HRR is a possible underlying cause of the
familial cancer predisposition, as a role in promoting