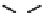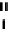Biology Reference
In-Depth Information
Ara-CMP
Ara-CTP
G
TCAGGT
NH
2
Excision of Ara-CMP
leads to resistance
N
AraC
O
O
O
N
O
G TCAGGT
O
P
O
-O
P
O
P
O-
O-
O-
O
HO
OH
Termination of DNA synthesis
induction of apoptosis
G TCAGGT
NH
2
dFdCTP
N
“Masked chain
terminator”
leads to higher
cytotoxicity
FC
O
O
O
N
O
G TCAGGT
-O
P
O
P
O
P
O
O-
O-
O-
O
F
dNTPs
OH
F
FC
A
G TCAGGT
FIGURE 5.13
Differences in the mechanism of chain termination by gemcitabine and ara-C. After incorporation into DNA, ara-CTP
terminates DNA synthesis directly at the site of incorporation while gemcitabine can be elongated by one additional nucleotide. The placement
of gemcitabine at the penultimate position is termed “masked chain termination” since the terminal nucleotide masks detection and removal of
gemcitabine by exonucleases or DNA repair enzymes.
existence of additional mechanisms of action and spec-
trum of activity against various solid tumors. In general,
the enhanced cytotoxic effects of dFdCTP are caused by
additional pharmacodynamic activities of the di- and
triphosphate forms of gemcitabine. These metabolites
inhibit various enzymes involved in the anabolism of
natural nucleosides. This inhibition leads to higher
intracellular concentrations of gemcitabine metabolites
that ultimately increases the probability of successful
incorporation of dFdCTP into DNA as well as RNA. In
particular, dFdCDP is a potent inhibitor of ribonucleo-
tide reductase (RnR) and this inhibition decreases the
levels of natural dNTP pools that are essential for
DNA synthesis during replication, repair, and recombi-
nation.
179
The inhibition of RnR by dFdCDP is the most
important one of these self-potentiating mechanisms as
RnR inhibition leads to depletion of dCTP, a potent feed-
back inhibitor of dCK. Relief of this feedback inhibition
leads to more efficient phosphorylation of gemcitabine,
thus generating higher intracellular levels of dFdCTP.
216
This activity is unique to gemcitabine as the closely
related analog, ara-C, does not alter dNTP levels by
inhibiting RnR.
235
Other reported activities of gemcitabine metabolites
include the inhibition of CTP synthetase
236
and the inhi-
bition of dCMP deaminase by dFdCTP.
237
Inhibiting
dCMP-deaminase by high concentrations of dFdCTP
reduces gemcitabine catabolism. The inhibition of
CTP-synthetase by dFdCTP leads to reductions in
rCTP pools which then allows for the incorporation of
dFdCTP into RNA to alter cellular RNA synthesis.
However, while it has been demonstrated that dFdCTP
can be incorporated into RNA,
238
the effects of this
modified nucleotide on cell viability as well as the
induction of apoptosis are not entirely clear.
Gemcitabine and ara-C can also inhibit the activity of
topoisomerase I, suggesting that induction of topoiso-
merase I-mediated DNA break formation can contribute
to the cytotoxicity of these nucleoside analogs.
239
Again,
the mechanisms accounting for cell death are slightly
different between the two analogs. While gemcitabine
and ara-C can trap topoisomerase I cleavage complexes,
the unique conformation of the ribose moiety of gemci-
tabine enhances the stability of topoisomerase I cleavage
complexes. The increased stability of the topoisomerase
I cleavage complex can generate DSBs by blocking the
movement of advancing proteins involved in replication
and transcription. Increasing the frequency of collisions
between replication and transcription complexes with
topoisomerase I cleavage complexes leads to an accumu-
lation of DSBs to cause cell death.
In addition to acting as a chain terminating nucleo-
tide, gemcitabine may also induce DNA hypermethyla-
tion of various gene promoters. In fact, it has been
suggested that treatment of cells with gemcitabine leads
to the epigenetic silencing of critical DNA repair