Environmental Engineering Reference
In-Depth Information
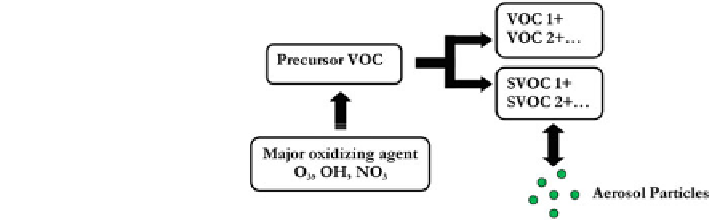
Fig. 3 Schematic overview
of SOA formation (adapted
from Dusek et al.
2000
)
aliphatics, aromatics) which are not as reactive and show limited activity in
presence of OH radicals. ROGs that have a double bond can be oxidized with
O
3
and NO
3
. Table
1
depicts the reactivity of ROG groups with these oxi-
dizing agents.
A few of these oxidation products are seen in particle phase due to their lower
vapor pressure such as polyols, dicarboxylic acids, amino acids and other such
multifunctional compounds (Saxena and Hildemann
1996
; Holes et al.
1997
;
Forstner et al.
1997a
,
b
; Limbeck and Puxbaum
1999
; Blando et al.
1998
; Rogge
et al.
1993
).
(b) Liquid Phase Oxidation
—
Most of the precursor gases emitted by anthro-
pogenic sources are water insoluble but their hygroscopicity increases with
aging. Fogs and clouds being rich in oxidizing agents leads to adsorption of
hygroscopic VOCs. Carboxylic acids, glyoxal, esters, and organo sulfur
compounds are the usual products of fog and cloud processing (Blando and
Turpin
2000
). Studies have revealed several mono and di-carboxylic acids
found in rain water and fog (Saxena and Hildemann
1996
; Blando and Turpin
2000
; Facchini et al.
1999
). Formaldehyde can also get collected in the fog
droplets (Facchini et al.
1992
). A recent study (Aumont et al.
2000
) suggested
that carboxylic acid formation is most dominated by VOC oxidation in
aqueous phase.
Table 1 Reactivity of ROGs towards oxidizing species in urban air
Organic species
Ozone
OH radical
NO
3
radical
10
−
23
10
−
11
10
−
17
Alkanes, Cycloalkanes
≤
(0.3
8)
×
≤
-
10
−
21
)
10
−
11
10
−
16
)
Oxygenated aliphatics
≤
(2.2
×
(0.2
6)
×
≤
(1.4
×
-
≤
(6
×
10
−
21
)
(0.1
-
6)
×
10
−
11
≤
10
−
17
Aromatics
2
×
10
−
18
to
1.5
×
10
−
15
(0.8
-
12)
×
10
−
11
6
×
10
−
17
Alkanes, Cycloalkenes and
other ole
ns
to
3
×
10
−
11
Units cm
3
molecule
−
1
s
−
1
at 289 K
Adapted from Grosjean and Seinfeld
1967









Search WWH ::

Custom Search