Environmental Engineering Reference
In-Depth Information
Table 1 Heating values and adiabatic flame temperatures of syngas mixtures
H
2
mole
fraction
CO mole
fraction
Mol. weight
(kg/kmol)
Heating
value kJ/
kg
Heating
value kJ/
kmol
Adiabatic
fl
ame
temp (
= 1.0) (K)
ϕ
0
1
28.0
10,101
282,814
2,394
0.2
0.8
22.8
12,428
283,365
2,382
0.4
0.6
17.6
16,130
283,892
2,378
0.5
0.5
15.0
18,943
284,139
2,377
0.6
0.4
12.4
22,933
284,368
2,378
0.8
0.2
7.2
39,539
284,684
2,382
1
0
2.0
141,794
283,588
2,387
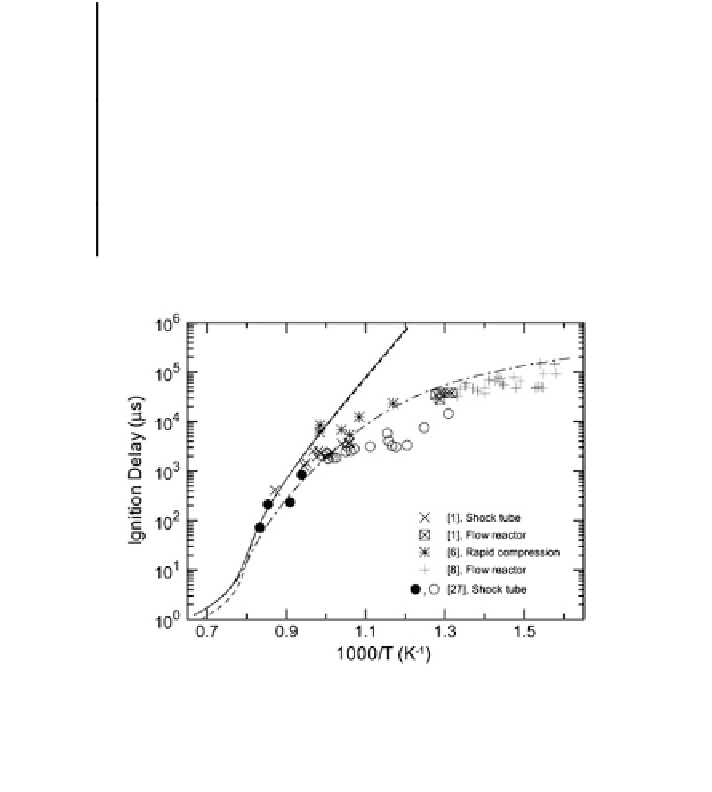
Fig. 2 Ignition delays for various syngas mixtures under different pressure and temperature
conditions. Filled and open circles correspond to strong and weak ignition events, respectively. All
experimental data have been normalized to 20 atm assuming
1 proportionality. Lines
correspond to ignition delay predictions using the Li et al. mechanism at 20 atm; the solid line
corresponds to the syngas mixture used in shock tube experiments
p
−
computations have often employed a homogeneous reactor con
guration (Aggar-
wal et al.
2011
). Figure
2
from Dryer and Chaos (
2008
) presents some measured
and predicted ignition delay data for different syngas mixtures. Additional data can
be found in K
é
romn
è
s et al. (
2013
) and other references cited above.
flame speed represents a fundamental property of a fuel-air mixture. It
is of critical importance with regard to burning rate,
Laminar
fl
fl
flame stabilization,
fl
ashback,
and blowout in practical systems. Laminar burning velocities for H
2
-
CO mixtures
have been measured using different systems, including
ame burner (Yan et al.
2001
), Bunsen burner (Natarajan et al.
2007
,
2009
), and expanding spherical
fl
at-
fl
ames
(McLean et al.
1994
; Prathap et al.
2008
; Kishore et al.
2011
). Figure
3
presents
measured and predicted laminar
fl
fl
flame speeds versus equivalence ratio and pressure,













Search WWH ::

Custom Search