Biomedical Engineering Reference
In-Depth Information
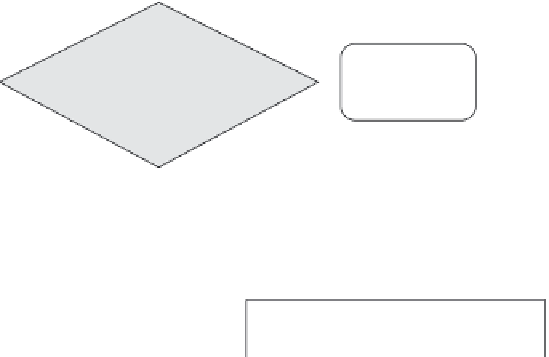
Optional initial screening*
for identification of
nanomaterials
Dispersing in fluid and
sample preparation using
identified “best practice”
SEM/TEM/AFM for
dispersion protocol
validation/screening
Best method based
on dispersion,
apparent size/
shape distribution
& image quality
Counting or
classifying
technique
SEM/TEM/AFM
particle counting
50%
n < 100 nm
No
Yes
Not
nano
Nano
* i.e., by ensemble methods (e.g. DLS)
or volume specific surface area (VSSA)
FIGURE 3.3
Draft of a tiered testing strategy that guides the user through a choice of tech-
niques to assess the size distribution by microscopic or nonmicroscopic techniques. (From
Brown, S.C. et al.,
Environ Health Perspect
, 121, 1282-1291, 2013.)
tree, including the dispersion protocol measurement method and error-propagating
evaluation software, will be delivered by the NanoDefine project (2013-2017).
3.6 CONCLUSION
It has been argued that nanomaterials should not be defined as long as we do not
have a mechanistic understanding of size-related hazards, because otherwise we
may fail to include some hazardous materials (Maynard 2011). Implicitly, such a
definition characterizes the term nanomaterial by small size and elevated hazard, but
how should one determine both elements by adopted methods if none precedes the
other? The regulators have decided not to strive for a risk-based definition, and have
instead established rather inclusive size-based definitions (Table 3.1). Nonetheless,
size criteria (Auffan et al. 2009) together with reactivity (Zhang et al. 2012) and
other triggers will be ingredients of QSARs that screen for hazardous materials
among the nanomaterials (compare Chapter 16).











Search WWH ::

Custom Search