Biomedical Engineering Reference
In-Depth Information
nongovernmental organization BUND,
*
99 products are itemized under the category
“contains nanomaterial: silver” out of a total of 1007 products claiming to contain
nanomaterials listed on this site: http://www.bund.net/nc/themen_und_projekte/
nanotechnologie/nanoproduktdatenbank/produktsuche/ (accessed on 10/25/2013).
Searching the global trade website www.alibaba.com for the terms “nano-material,
nano-technology, nano-particles” yields >70,000 products, whereas a specific search
for “nanosilver, nano-silver” yields >7400 products, confirming that a multitude of
products claiming to contain nanosilver have found their way into the global market
(Alibaba 2013).
19.2 INTRODUCTION
Consensus has been reached that risks to human health from manufacture and use
of nanomaterials including nanosilver can, in principle, be characterized using the
established risk assessment paradigm. This approach to risk assessment is based on
four fundamental steps: identification of the hazard potential of a product and its
components, characterization of the relevant hazards namely their dose-response
relationships, assessment of exposures to the product and/or its critical components,
and finally, a characterization of the likely risk by integration of the information
on hazard and exposure characteristics (Figure 19.1). A more in-depth description
of this approach, as well as recommendations for additional considerations aim-
ing to improve its utility for risk assessment and the technical analyses supporting
risk assessment, has been provided by the National Research Council (NRC) (NRC
2009). With regard to application of the risk assessment paradigm to nanomaterials,
a detailed analysis of the applicability of this approach and its (default) assumptions
by the Organization for Economic Co-operation and Development (OECD) Working
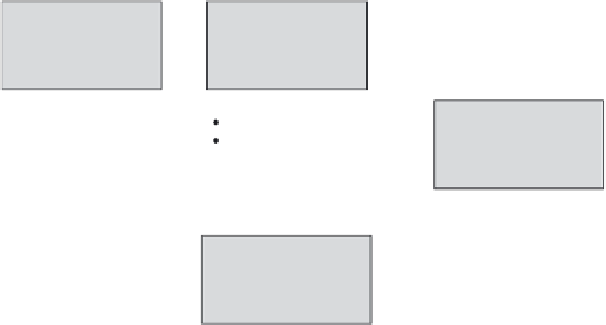
Hazard
identification
Identification of
potential adverse
effects in relevant
mammalian species
Hazard
characterization
Characterization of
the dose-response
relation for adverse
effects
Risk
characterization
Definition of
NOAEL,
Point of departure, or
Reference value
Risk characterization
by comparison of
dose-response to
exposure information
Calculation of
Risk characterization ratio
(exposure ÷ reference value)
Margin of exposure
(exposure ÷ NOAEL),
etc.
Exposure
assessment
Definition of relevant
exposure routes,
time-frames and
extent of exposure(s)
FIGURE 19.1
The basic four steps of the risk assessment paradigm.
*
Bund für Umwelt und Naturschutz.










Search WWH ::

Custom Search