Biomedical Engineering Reference
In-Depth Information
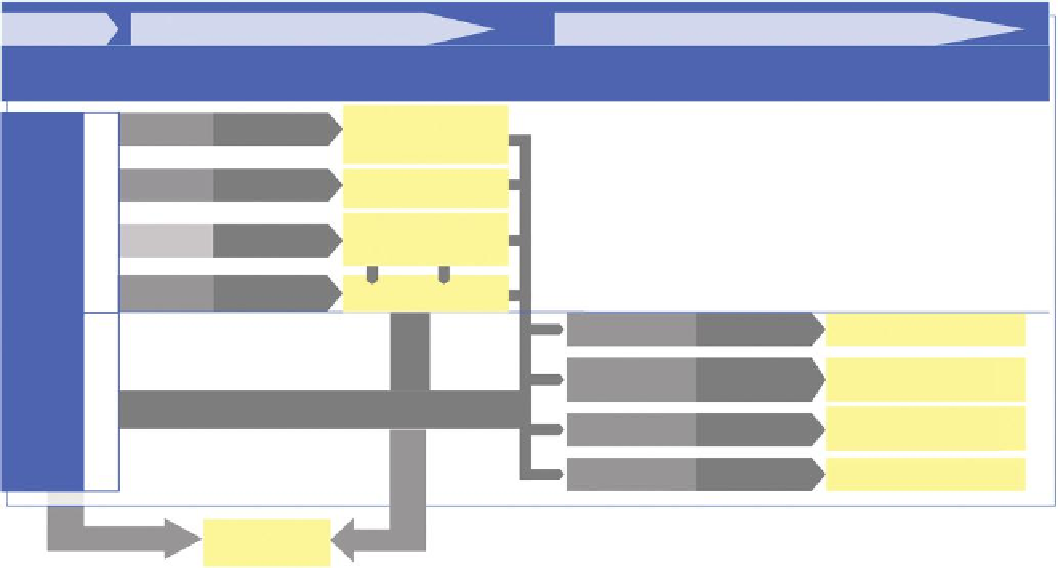
TIER 1
TIER 2
TIER 3
Toxicity
domain
Decision-
making process
Test options
Toxicity domain
Decision-making
process
Test options
- EpiDerm
Local
tolerance
- BCOP and EpiOcular
-
in vitro
sensitisation strategy
or LLNA
in vitro
micronucleus test
and HPRT assay
Judgement
In vitro
genotoxicity
Judgement
- Absorption/dermal
penetration
- Accumulation/persistence
- Barrier crossing
ADCE
Judgement
Short-term
toxicity
Short-term toxicity (incl.
inflammation, cytotoxicity)
Judgement
in vivo
genotoxicity
Judgement
in vivo
tests
in vivo
repeated toxicity study
incl. kinetics
(absorption, tissue accumulation)
Developmental/fertility testing
incl. kinetics
(absorption, tissue accumulation)
Organ toxicity
(repeated-dose)
Judgement
Reproductive toxicity
Judgement
Carcinogenicity
Judgement
Establish general concept for NM
Grouping/
waiving
FIGURE 16.4
Integrated approach for the hazard testing and assessment of nanomaterials (NM) addressing speciic concerns for an individual NM.
(Reprinted with permission from Oomen AG et al. (2013), Concern-driven integrated approaches to nanomaterial testing and assessment, Report of the
NanoSafety Cluster Working Group 10,
Nanotoxicol
28 May 2013, epub ahead of print.)
Search WWH ::

Custom Search