Biomedical Engineering Reference
In-Depth Information
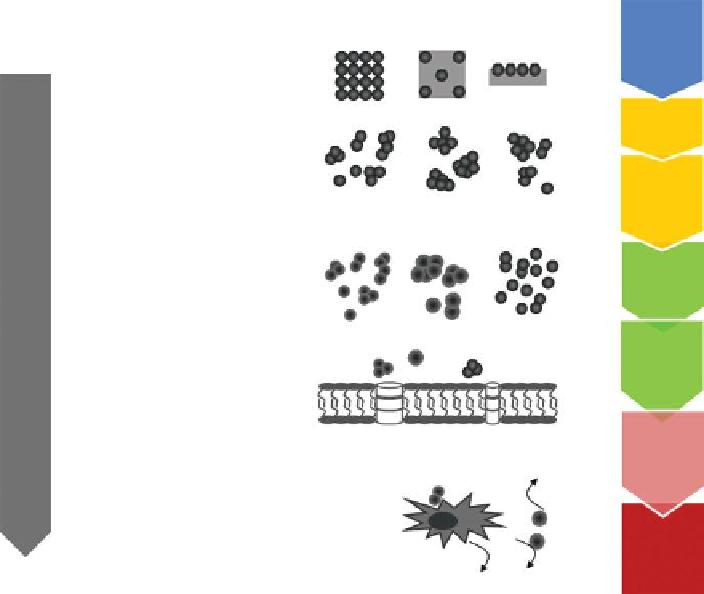
Powder
Embedded in matrix
or on surfaces
Changes of phys.-chem. properties
Aerosol
Suspension
Deposition in the lung,
Alveolar, intestinal, dermal absorption
Surface coating changes
Agglomeration, deagglomeration
Crossing of biological barriers
Tissue distribution,
Intracellular distribution
Inflammation
Catalysing formation of reactive compounds
Ion release
Direct interaction with cellular structures
M
+
Organ toxicity
ROS, RS, ...
FIGURE 16.2
Source-to-adverse-outcome pathway of nanomaterials. (Landsiedel, R.
et al.: Testing metal-oxide nanomaterials for human safety.
Adv. Mater
. 2010. 22. 2601-2627.
Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.)
exposure refers to the level and physicochemical form of a NM at the site of action in
the organism. This includes biokinetics, of which transport across barriers (external
barriers such as skin, lung, and gastrointestinal tract and internal barriers such as
blood-brain, blood-testis, and placenta) and modification in the body are the most
critical for NMs. If, for example, two NMs exert the same early biological effects
at a similar degree, but internal exposure for one NM is much lower, the potency of
the apical effect will be different. Furthermore, tissue distribution may be different
between NMs and one NM may reach a target tissue to initiate a tissue-specific effect,
whereas the other NM may not. These aspects are referred to as “uptake in the body,”
“modification in the body,” and “distribution in the body” in Figure 16.2. Hence,
information on various steps of the source-to-adverse-outcome pathway is required.
To date, no NM effects have been reported that have not yet been observed with any
other substance or particle. It therefore seems generally feasible that toxicity of NMs
can be investigated with standard testing methods used for conventional chemicals,
adapted to NM-specific requirements for test sample preparation and characteriza-
tion and test interference controls, as also recommended by the OECD's Chemicals
Committee, a parent body to the WPMN (OECD 2013). For in vitro toxicity tests,
Search WWH ::

Custom Search