Biomedical Engineering Reference
In-Depth Information
directly or by providing information that is also relevant for grouping, read-across,
ranking, and other methodologies as a part of IATA to fill data gaps that will allow
waiving of testing. In the ideal situation, the testing strategy drives a grouping
approach in order to efficiently and effectively assess risk.
In the following, an overview of NM pathways from use and release to apical
toxic effects is given and ongoing developments with regard to the testing and group-
ing of NMs are presented (Figure 16.1). Efficient and effective safety assessment of
NMs should be concern-driven; that is, they should start out by identifying relevant
concerns and critical lifecycle stages, based on NM exposure and use scenarios,
physicochemical characterization of the NMs (related to exposure, uptake, biodistri-
bution, and hazard), estimation of changes in physicochemical characteristics during
the lifecycle, and already available toxicological information. Recognized concerns
will not only influence the IATA but also drive the grouping process.
Whereas the deliberations on IATA and NM grouping are not directly related to
the existing legislation, they do stand in line with EU guidance on NM safety testing
(Hankin et al. 2011; ECHA 2012a). For example, alternative methods are propagated
in REACH (Annex XI), if sufficiently documented, scientifically found, and appli-
cable for risk assessment and classification and labeling. However, the material prop-
erties and exposure situation for NMs are often complex and the scientific foundation
and their applicability for risk assessment are not yet established. The present delib-
erations are a first approach to assess the complexity for NMs and how to deal with
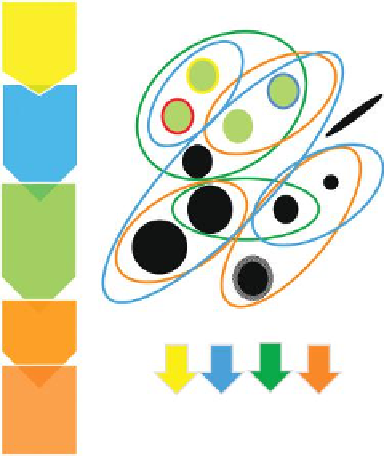
Toxicological testing
FIGURE 16.1
Using the
Source to Adverse Outcome Pathway
for grouping concepts as
key elements of testing strategies. The circled figures represent potential different groups of
nanomaterials, either based on material properties, exposure information, kinetic behavior,
and/or effects. (Modified from Oomen AG et al. (2013), Concern-driven integrated approaches
to nanomaterial testing and assessment, Report of the NanoSafety Cluster Working Group 10,
Nanotoxicol
28 May 2013, epub ahead of print.)
Search WWH ::

Custom Search