Biomedical Engineering Reference
In-Depth Information
9
30 min
2 h
48 h
6
3
0
0,01
0,1
1
10
100
Size (µm)
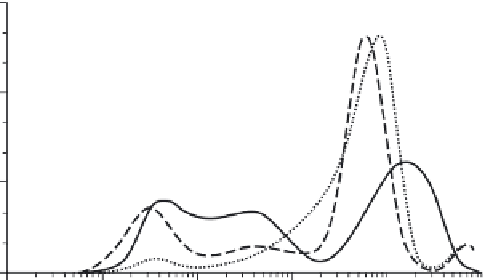
FIGURE 15.10
Size distribution of a nanocomposite used in sunscreen (Figure 15.9) aged
in water for different times. (Reprinted from
Environ. Pollut
., Labille, J. et al., Aging of TiO
2
nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aque-
ous environment, 3482-3489, Copyright 2010, with permission from Elsevier.)
50 nm
2 nm
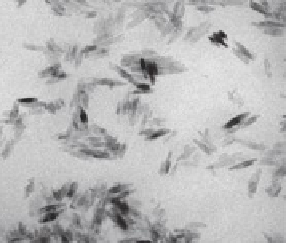
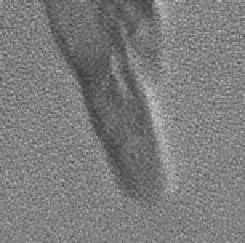
FIGURE 15.11
Transmission electron microscopy image of the stable suspension of
a sunscreen aged in water. (Reprinted from
Environ. Pollut
., Botta, C. et al., TiO
2
-based
nanoparticles released in water from commercialized sunscreens in a life-cycle perspective:
Structures and quantities, 159, 1543-1548, Copyright 2011, with permission from Elsevier.)
the rest was agglomerated and sedimented rapidly (Figure 15.10). The stability of the
suspension was determined by the properties of the aluminum oxide coating.
Botta et al. (2011) extended this work and studied not the pure nanocomposites
but the release from four commercially available sunscreens. The results showed that
a significant fraction of nano-TiO
2
was released from all sunscreens when they were
put into water. A stable colloidal phase was formed which contained between 16 and
38% of the mass of the sunscreen and 1%-30% of the total TiO
2
. About 10%-30%
of the particles in this suspension were smaller than 1 µm, however, the volumetric
fraction below 100 nm was almost negligible. A TEM investigation of the stable
suspension showed rods of nano-TiO
2
with approximately 10 × 50 nm size that were
agglomerated to larger particles (Figure 15.11).




Search WWH ::

Custom Search