Biology Reference
In-Depth Information
found by TCSPC [
35
]. The major conformation with the longer t
Fl
¼
2.8 ns is
associated with a conformation, where Y145 is buried within the chromophore
pocket and H148 is exposed to the solvent. In the minor conformation, however, the
occupation of the interior by both hydrophobic amino acids is reversed leading to a
reduced t
Fl
¼
0.6 ns. Thus, by the substitution H148D, where the hydrophilic
aspartate is exposed to the solvent at neutral pH, a CFP called Cerulean with a
distinctly longer but not monoexponential t
Fl
is developed [
62
]. Molecular dynamics
simulations are performed to understand the effect of various amino acid replacements
on the lifetime [
58
,
73
]. The lifetime differences between CFP and Cerulean are
associated with the preferential stabilization of a completely planar structure in the
latter (see Fig.
3c
).
At lower pH values, however, a conformation of D148 is observed where this
residue is bound through a hydrogen-bridge to the chromophore and, thus, stabiliz-
ing the latter in an isomeric rotamer [
65
]. Concomitantly, a distinct reduction of t
Fl
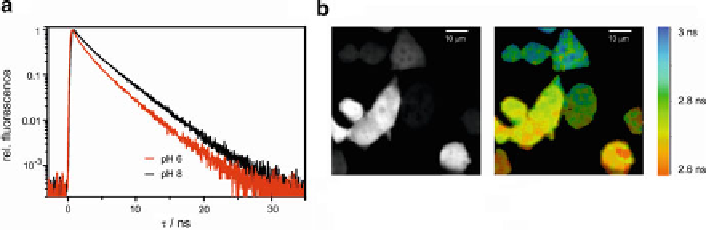
by 40% under acidic conditions is observed (Fig.
8a
)[
59
]. CFPs can thus be used as
FLIM-indicator revealing cellular acidification with spatial resolution (Fig.
8b
).
The occurrence of multiple conformational states in CFPs challenges the assign-
ment of individual lifetime components to crystallographically defined structures
and backs lifetime heterogeneity [
48
,
59
]. However, it should be mentioned that a
higher F
Fl
due to H148D was confirmed. Most recently, introduction of the smallest
amino acid at position 148, i.e., mutation H148G, as well as bulkier amino acids at
V224 (V224L, V224R) leads to prolonged t
Fl
>
4 ns thus approaching t
rad
[
61
].
This goes together with a maximal fluorescence quantum yield near 1.
3.3 Green and Yellow Fluorescent Proteins
The most exhaustive investigations of the dependence of t
Fl
on structural effects
are performed on proteins with Y66 as central aromatic amino acid as part of the
Fig. 8 ECFP as pH-sensor for FLIM. (a) ECFP fluorescence decay (l
exc
¼
440 nm; l
obs
¼
480 nm)
is shorter at lower pH (apparent p
K
a
820 nm)
of HEK293 cells expressing ECFP (
left
: intensity;
right
: fluorescence lifetime). The extracellular
solution has a pH of 6.5 and 10
¼
6.5) (b) Twophoton fluorescence images (l
exc
¼
M of the K
+
/H
+
m
ionophore nigericin leading to lowering of
intracellular pH in part of the cells
Search WWH ::

Custom Search