Biology Reference
In-Depth Information
1520
1570
1620
1670
1720
wavenumber
Fig. 7 Time-resolved IR spectroscopy of avGFP in D
2
O recorded 2, 6, 10, 30, 100 and 200 ps
after excitation at 400 nm. The direction of increasing/decreasing absorption is indicated
solution [
97
,
99
]. Although the main bleach bands seen in avGFP can be assigned to
the chromophore, the temporal evolution of the shape of the transient IR spectrum
suggests additional underlying contributions from protein modes. The origin of some
of these protein modes has been revealed by mutagenesis [
99
].
The proton-transfer dynamics and the site of the proton acceptor are revealed
in two time-dependent bands - a bleach that develops as a function of time at
1560 cm
1
and a new transient absorption above 1700 cm
1
(Fig.
7
). These bands
evolve on the same picosecond timescale and are assigned to the conversion of a
carboxylate to a carboxylic acid. Inspection of the structure of avGFP suggests that
this transformation may be assigned to protonation of the residue E222, which, as
first shown in the structural studies of Brecj et al. [
95
], is connected to the proton
donor by a proton wire via a structural water molecule and the S205 residue. Thus,
transient IR spectroscopy confirms the proposed assignment of the E222 residue as
the proton acceptor. A comparison of the fluorescence decay time with the vibra-
tional dynamics shows that donor decay and acceptor protonation occur simulta-
neously, suggesting a concerted mechanism for proton motion, or at least that any
intermediates states are very short lived [
96
]. The role of the E222 residue has been
supported by studies of mutants, polarisation-resolved measurements, isotopic
labelling and observations over a wider spectral range [
97
,
100
]. The non-exponen-
tial dynamics observed in both fluorescence and transient IR suggest dispersive
kinetics, which observations over a wider spectral range were able to assign to side-
chain disorder leading to different proton-transfer rates [
100
].
These studies of the structure and dynamics of the proton relay reaction have
provided a rather detailed picture of the photophysics of avGFP. They also suggest
the use of GFP as a model system for the study of proton-transfer reactions in
proteins. Proton-transfer is one of the key steps in many biochemical reactions, and
transfer along proton wires has often been invoked in biochemical mechanisms
[
101
-
103
]. The unique ESPT reaction in GFP permits the measurement of the real-time
dynamics of such proton-transfer reactions, following photoinitiation with an ultrafast
laser pulse. The comparison between transient IR and fluorescence data already



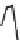





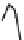
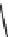
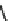
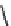




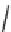



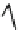


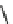
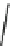


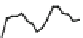






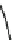





































































































Search WWH ::

Custom Search