Biology Reference
In-Depth Information
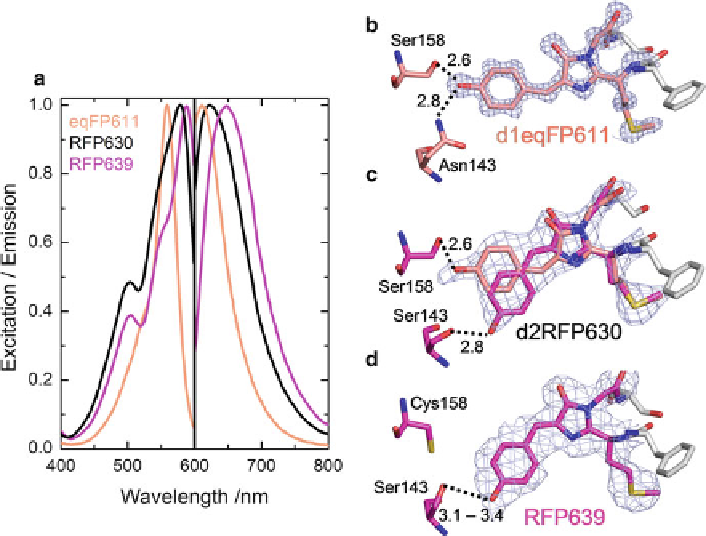
Fig. 4 Red-shifting the absorption and emission of eqFP611 by rational engineering. (a) Excita-
tion and emission spectra of d1eqFP611, d2RFP630, and RFP639. (b) Stick representation of the
d1eqFP611 chromophore (PDB code 3E5T). (c) Stick representation of the d2RFP630 chromo-
phore (PDB code 3E5V). (d) Stick representation of the RFP639 chromophore (PDB code 3E5W)
a reorganization. Mutational studies have identified a hydrogen bond between
Glu16 and the chromophore as the major cause for the red shift [
25
]. The temporal
shift of the fluorescence emission in mPlum was explained by a time-dependent
interaction between Glu16 and the excited state of the chromophore, with the
glutamic acid side chain performing a rotation in response to the modified charge
distribution in the excited state.
Fluorescent Timers
Fluorescent timers, initially introduced by Terskikh et al. in 2000 [
71
], are FPs which
change their emission wavelengths as a function of time. Subach et al. used mCherry
as a template to generate monomeric fluorescent timers [
72
]. Maturation of their
chromophores proceeds via a blue intermediate with a single C
bond of the
chromophore-forming Tyr. Oxidation of this particular bond is the last step in the
maturation of the red chromophore. The timer property is based on a delayed oxida-
tion of this bond. Site-directed mutagenesis revealed that, in mCherry, the delay was
achieved by replacing Lys70 and Leu83 by Arg and Trp, respectively [
72
].
a
-C
b
Search WWH ::

Custom Search