Biology Reference
In-Depth Information
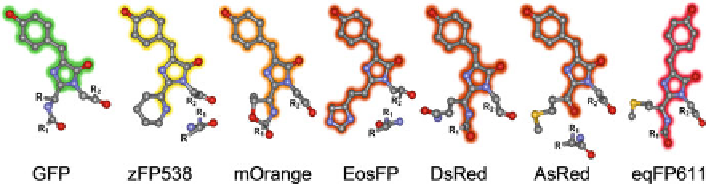
Fig. 2 The GFP chromophore and its autocatalytically produced variants (adopted from [
24
])
proteins such as DsRed [
22
] and eqFP611 [
23
], the single bond between the amide
nitrogen and the C
atom of the first of the three chromophore-forming amino acids
is oxidized to an acylimine group in-plane with the p-HBI chromophore (Fig.
2
).
Consequently, the conjugated
a
-electron system of the resulting 2-imino-5-(4-
hydroxybenzylidene)-imidazolinone chromophore is further extended, and the
emission shifts bathochromically to ~600 nm. The delocalized
p
-electron system
may extend even further to include the carbonyl group of the preceding amino acid
in FPs emitting further to the red [
25
].
In mOrange, a monomeric DsRed variant emitting at 562 nm, an oxazole
heterocycle (Fig.
2
) is created from the side chain of Thr66, the first amino acid
of the chromophore triad in this protein [
26
]. Its hydroxyl group apparently attacks
the preceding carbonyl carbon. The yellow zFP538 from
Zoanthus
sp. likewise
features a third heterocycle, formed by ring closure of a lysine side chain [
27
].
Presumably, the terminal amino group of the lysine side chain attacks the reactive
N
p
acylimine bond and, concomitantly, the protein backbone is cleaved, leav-
ing the N-terminal fragment with a terminal carboxamide (Fig.
2
). The smaller red
shift compared with the acylimine of DsRed has been rationalized by the less
effective charge delocalization by the cyclic imine [
27
]. In AsRed, the mutant
Ala143Ser of asFP595 from
Anemonia sulcata
[
28
], the X-ray structure analysis
revealed that chromophore maturation is accompanied by a break in the polypeptide
chain [
29
-
31
], and a carbonyl group, generated by hydrolysis of the intermediately
formed acylimine group, extends the conjugated
¼
C
a
p
-electron system of the
p
-HBI
chromophore (Fig.
2
).
In addition to these spontaneously occurring chemical alterations of the green
chromophore, photoinduced modifications can occur, as will be discussed in detail
in Sect. 3.5.2.
2.4 Quaternary Structure
GFP has only a weak tendency to form dimers; the dissociation coefficient has been
estimated as 100
M[
32
]. The homodimeric interface includes a small patch of
hydrophobic residues (Ala206, Leu221, Phe223) as well as a large number of
m
Search WWH ::

Custom Search