Biology Reference
In-Depth Information
molecule emission intensity over time, or, when pulsed excitation is used, to
determine the emission lifetimes of single emitters. Sensitive CCD cameras in
combination with a dispersing element such as a grating or a prism are used to
detect full fluorescence spectra of a single molecule.
To characterize the photophysical properties of VFPs, the fluorescent proteins
are usually immobilized by embedding them at very high dilutions (~10
11
M VFP
in polymer solution) in a very thin film of a nonfluorescent polymer. In this way, the
proteins are separated laterally, so that on average less than one protein can be
found within the diffraction limited observation volume. The immobilization in the
polymer ensures that the VFPs are not diffusing out of the detection volume during
the characterization, which typically takes some seconds per single protein.
3.2 Single VFP Intensity Trajectories
The intensity trajectory of a single emitter describes the evolution of its emission
intensity over time. The majority of single fluorescent protein studies analyze the
intensity trajectory, gaining insights into processes affecting the brightness of the
single molecule, such as changes in molecular orientation or quantum yield,
transient transitions into dark states (often called “blinking”), or photobleaching.
Many of these phenomena can be observed for any single emitter including
chemical fluorophores, quantum dots, or the VFPs discussed here. Since the inten-
sity trajectory is the most accessible parameter on the single molecule level, the
analysis of single VFP intensity trajectories already started in 1997 [
58
]. Dickson
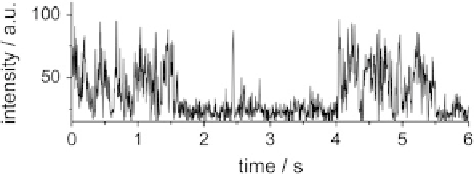
et al. recorded intensity trajectories that showed evidence of repeated cycles of
fluorescent emission and transitions into dark, nonemitting states on a timescale
of several seconds, behavior clearly not observable in ensemble studies due to
the averaging of the emission from different molecules. Comparable blinking of the
emission was observed from different fluorescent proteins [
59
-
62
] (Fig.
2
). The
duration of the on-times was found to decrease with increasing excitation power
[
35
], which points toward an excitation-driven, photoinduced transition to the dark
state. The nature of these dark states can vary, including changes in the protonation
state of the chromophore [
58
,
62
,
63
], efficient
cis
/
trans
photoisomerization that
quenches the fluorescence [
64
], or rearrangements in the chromophore environment
defined by the protein backbone leading to other effective deactivation pathways.
Fig. 2 Typical single
molecule intensity
trajectories of the enhanced
Green Fluorescent Protein
(EGFP). The emission is
interrupted by numerous dark
intervals
Search WWH ::

Custom Search