Biology Reference
In-Depth Information
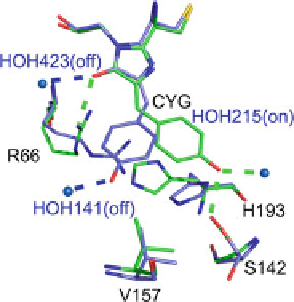
Fig. 14 Comparison of the
on-state (
blue
) and off-state
(
green
) structures of Dronpa
in the chromophore vicinity
(reproduced with permission
from Andresen et al. [
80
])
Dronpa, photoswitching of asFP595 also involves protonation change of the chromo-
phore [
94
]. However, in asFP595, the imidazolidinone nitrogen is proposed to form
a zwitter-ionic species, on the basis of theoretical work [
63
,
64
](Fig.
15
).
Several computational and simulation studies have focused on the photoswitch-
ing dynamics of “RSFPs” [
63
,
64
,
90
,
95
,
96
]. Sch
afer et al. [
63
,
64
] proposed that
the chromophore is present as a zwitter-ion in the
trans
form, and that ESPT
proceeds from the imidazolidinone nitrogen (rather than the phenolic oxygen). In
GFP, the zwitter-ion was shown not to be present. For the free chromophore model
compound ethyl 4-(4-hydroxyphenyl)methylidene-2-methyl-5-oxoimidazolacetate,
Bell et al. [
97
] determined a pKa value of 8.0 for protonation of the imidazolidinone
nitrogen [
97
] and showed from resonance Raman spectroscopy that in GFP at pH
8.0, where a mixture of anionic and neutral species is present, the position of the
Raman bands exclude the possibility of cationic and zwitter-ionic forms [
97
]. In the
case of asFP595, an acidic carboxylate from Glu215 is in hydrogen-bonding contact
with the imidazolidinone nitrogen [
82
,
84
,
85
]. QM/MM simulations hitherto
suggest that the zwitter-ion is the dominant species [
63
,
64
], but this is yet to be
confirmed by vibrational spectroscopy. Schafer et al. [
63
] propose that ESPT
deactivates the otherwise fluorescent zwitter-ionic
trans
chromophore, explaining
the low fluorescence quantum yield [
63
,
64
]. Indeed, ultrafast spectroscopy was
used to determine the lifetime of the excited state, and found a dominant decay
component of 320 fs, corresponding to the low fluorescence quantum yield
<
10
4
[
98
]. The existence of a dark zwitter-ionic state was proposed early on from semi-
empirical calculations of the GFP chromophore [
99
], providing a direct explanation
for “blinking” behaviour observed at the single molecule level [
100
,
101
]. Semi-
empirical calculations predicted that in GFP in the ground state the zwitter-ion is not
populated [
102
].Furthermore, the calculated excited-state free energy levels show
the expected increased acidity of the phenol oxygen and increased basicity of the
imino nitrogen [
102
]. This agrees with a picture of charge migration in the excited
state typical of photoacid behaviour, and counters the results from Sch
€
afer et al.
[
63
,
64
] who propose deactivation of the excited zwitter-ion state via proton transfer.
€
Search WWH ::

Custom Search