Biology Reference
In-Depth Information
2 Photoconversion of A. victoria GFP
2.1 Discovery and UV/VIS Spectroscopic Investigations
of Photoconversion of GFP
Photoconversion of GFPwas discovered shortly after the cloning of the
gfp
gene [
1
,
7
].
Photoconversion is found to occur in a range of different optical regimes, using UV
and visible light illumination, and with femtosecond and nanosecond pulses as well as
continuous illumination. The report by Chalfie et al. [
1
] on recombinant expression of
the gfp gene included a note: “Indeed, the fluorescence produced by 450- to 490-nm
light appeared to be more intense after brief photobleaching by 340- to 390-nm light”
[
1
]. A first spectroscopic characterisation of this photochromic reaction appeared in
1995 from the laboratory of Roger Tsien [
7
](Fig.
5
) This paper showed the fluores-
cence changes with UV illumination at 280 nm. Cubitt et al. [
7
] proposed photo-
isomerisation as a mechanism and found the spectroscopic changes to be irreversible.
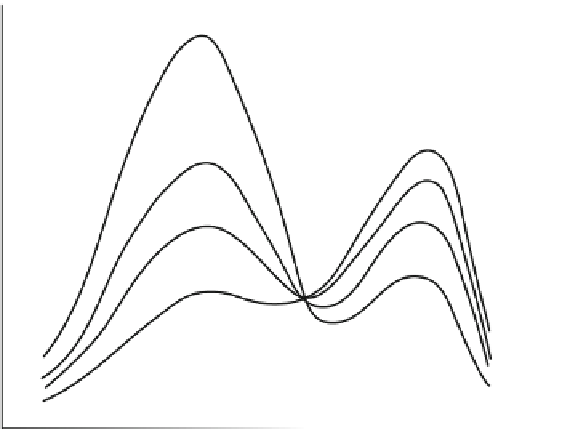
The fluorescence intensity changes in Fig.
5
indicate the occurrence of both photo-
bleaching and photoconversion under the conditions used, considering that at pH 8.0
a
4
q
+5
0 min
3
q
+5
13 min
2
q
+5
23 min
40 min
1
q
+5
0
q
+0
325
350
375
400
425
450
475
500
525
Excitation wavelength (nm)
Fig. 5 First spectroscopic demonstration of photoconversion of GFP. Reproduced with permis-
sion from Cubitt et al. [
7
]. Selected figure caption: “Behavior of wild-type green fluorescent
protein (GFP) upon progressive irradiation. GFP samples were illuminated at 280 nm from a xenon
lamp and monochromator. Wild-type GFP suffered photoisomerization, decreasing the excitation
amplitude at 395 nm while increasing the amplitude at 475 nm. This effect was not reversible upon
placing the GFP in the dark” [
7
]










Search WWH ::

Custom Search