Biology Reference
In-Depth Information
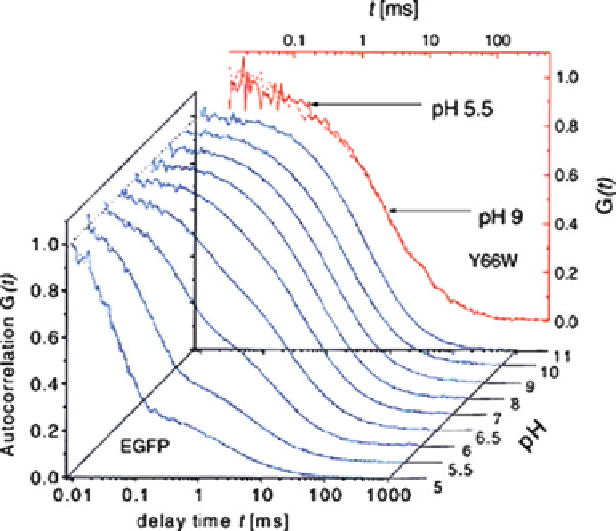
Fig. 1 The autocorrelation of the chromophore fluorescence of the GFP mutant Y66W and of
EGFP. For Y66W, the spectra at pH 5.5 and at pH 9 are almost identical. The autocorrelation decays
with a time constant of about 10 ms. For EGFP, the autocorrelation decays much faster at lower pH
indicating a proton equilibrium with bulk solution. Figure reprinted from [
12
] with permission
3 pH-Jump Experiments
The picture obtained from FCS measurements is complemented by experimental data
that analyzed changes of the chromophore photophysics following an external pH
jump of the medium [
15
]. When exposed to laser light, small chemicals known as
“caged protons” undergo ESPT and release protons into the medium. In this way, a pH
jump of the medium fromhigh to low pH can be generated within a laser flash of 10-ns
duration. Following the pH jump, the following elementary processes will occur: (1)
free diffusion of protons in the bulk solvent, (2) binding of protons frombulk solvent at
the protein/water interface, (3) proton flow through a hydrogen-bonded network from
the interface to neighboring titratable groups or water molecules around the buried
chromophore, and (4) rearrangement of the side chains around the GFP chromophore,
as well as of the chomophore itself, to enable PT to the deprotonated chromophore.
The combination of all these factors will then lead to the pH-jump-initiated fluores-
cence changes at the chromophore site that can be detected spectroscopically.
NMR experiments and computer simulations have determined the diffusion time
of excess protons in water as
D
10
5
cm
2
s
1
. In a simulation study of an
acetic acid molecule immersed into a water box of 2.4-nm dimensions [
16
], we
¼
9.3
Search WWH ::

Custom Search