Biology Reference
In-Depth Information
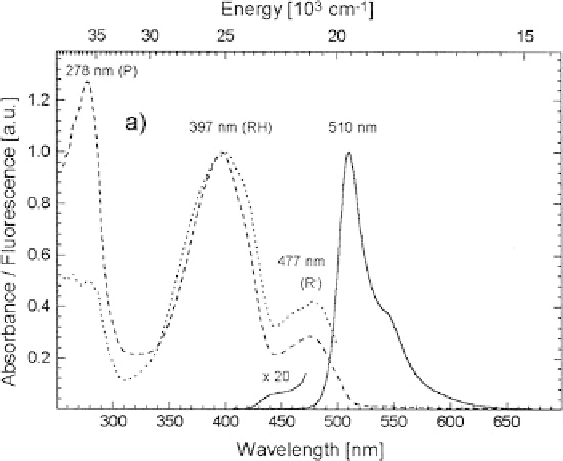
Fig. 3 Room temperature absorption (
dashed line
), fluorescence excitation (
dotted line
) and
emission spectra (
solid line
)of
av
GFP, at room temperature and pH 8.0 (adapted from Kummer
et al. [
15
])
397 nm and a minor band at 477 nm, due to absorption of the chromophore. Their
relative height depends on proton concentration: increasing pH above 11, the minor
lower-energy band increases at the expense of the higher-energy band. Vice versa,
at pH below 4 the minor energy band is completely depleted [
16
]. The relative
population in Fig.
3
is rather constant between pH 6 and 11. This behavior arises
from the ground-state equilibrium between two states of the chromophore, differing
in protonation of the phenolic group from Tyr66. The phenolic oxygen of the
chromophore is protonated in the state absorbing at 397 nm (RH in the following)
and deprotonated in that absorbing at 477 nm (state R
). It is commonly accepted
that the two other possible protonation sites in the chromophore, i.e., the nitrogen
and the carbonyl oxygen of the imidazolinone, are deprotonated in both
the absorbing states, thereby giving an overall neutral chromophore in state RH
(GFPn in Fig.
2
) and anionic in state R
(GFPa) [
17
].
Excitation of state RH leads to a fluorescence spectrum peaking at 510 nm
(Fig.
3
), with a rather high quantum yield of 0.79. State R
yields a similar
fluorescence spectrum, slightly blueshifted and peaking at 503 nm (not shown). In
both cases, fluorescence comes from emission of the singlet excited state of the
anionic chromophore. Excitation of the neutral chromophore results in ultrafast
(4 ps) excited state proton transfer (ESPT) and subsequent emission of the anionic
form [
18
]. The ESPT acceptor has been identified in (deprotonated) Glu222 [
18
,
19
].
Although the anionic chromophore is the emitting species in both states, the
configuration of the surrounding residues is different and the decay time of the
Search WWH ::

Custom Search