Biology Reference
In-Depth Information
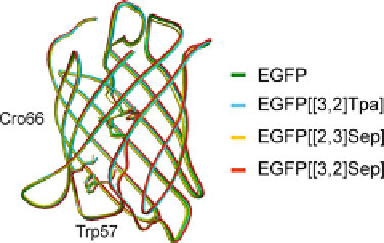
Fig. 12 Overlay of the amino
acid backbone as revealed by
crystal structure analysis of
EGFP and its chalcogen-
containing variants.
Expression of EGFP in
presence of [3,2]Tpa, [2,3]
Sep and [3,2]Sep results in
proteins with identical overall
topology
4 Applications and Further Development of Autofluorescent
Proteins Using NCAAs
Besides the use of NCAAs for structural analysis as discussed above, especially Sep
derivatives are beneficial for X-ray crystallography as they can help to overcome
problems in phase calculation [
69
]. But the highest potential of fluorescent protein
variants with NCAA lies doubtlessly in the application of their changed fluorescent
characteristics. A prominent example is the fusion protein of golden fluorescent
protein and human annexin A5 (GdFP-anxA5) as novel apoptosis detection tool as
recently reported by Kurschus and co-workers [
70
].
This approach made use of the extremly red-shifted fluorescence emission
(l
max,em
¼
574 nm) and the 1:1 ratio of fluorophore (GdFP) and lipid binding
protein (anxA5). Both are indeed improvements, as on the one hand fluorescence
markers with orange/red emission are of special interest because the autofluores-
cence background of the cell is low in this spectral region. On the other hand, the
defined label ratio is superior to commercial fluorescence labeled anxA5 as this is
often a mixture of heterologous products because of the chemical reaction
employed for labeling. Very often Lys residues are labeled which, unfortunately,
are partially in the vicinity of the binding domain of anxA5. Thus binding may
interfere with fluorescence [
71
,
72
]. These limitations can be circumvented by
using a fusion protein; however, GFP and its classical derivatives have their
emission maximum at
530 nm and the more red-shifted
ds
Red and its family is
not suitable for general applications as they still tend to oligomerize and aggregate.
In contrast, GdFP is almost as red-shifted as
ds
Red and exhibits an extraordinary
thermostability and keeps its monomeric state even after long-term storage at 4
C
[
70
]. Moreover, its approximately twofold higher stability to pH in the range of
5.5-7.0 when compared to anxA5-FITC provides the possibility for applications at
lower pH values. Furthermore, the 4-amino group of (4-Am)Trp, which is part of
the GdFP chromophore and replaces the single Trp residue in anxA5 upon SPI
expression, does not affect lipid binding of the fusion protein. Therefore, GdFP-
anxA5 is suitable for the quantification of apoptotic and necrotic cells and, in
addition, its use in combination with other dyes in flow cytometry or fluorescence
<
Search WWH ::

Custom Search