Biology Reference
In-Depth Information
the
cis
/
trans
isomerization of the adjacent proline residues [
60
]. In addition, the
substitution pattern of the Trp57's indole ring has also a great influence on the
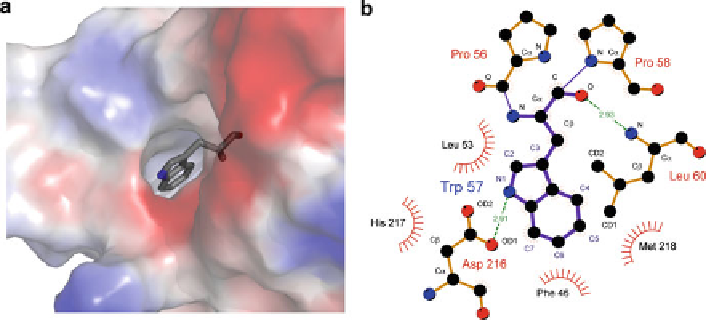
folding and stability of EGFP and ECFP. A closer inspection of the ECFP crystal
structure (1OXD) revealed the accommodation of Trp57 in the minicore cavity of
the protein with C4-C7 buried in the hydrophobic core, whereas N1 and C2 are
solvent exposed.
However, the observation that both (7-Aza)Trp and (4-Aza)Trp gave signifi-
cantly high amounts of insoluble protein cannot be explained solely by spatial
requirements. Indeed, C7 of Trp57 in ECFP is in interaction distance to several
atoms in the vicinity for instance with backbone nitrogen and oxygen atoms (Fig.
8
).
Introduction of nitrogen at C7 means an additional free electron pair at this site,
which may result in repulsive interactions [
59
].
It is well known that various Trp derivatives substituted at position 4 could be
incorporated into EGFP and ECFP including (4-Am)Trp and (4-Me)Trp [
11
].
These data as well as the crystal structure confirm that there is enough space
in the vicinity of C4 of Trp57. But why does (4-Aza)Trp incorporation result
in insoluble protein? It seems that the markedly increased hydrophilicity of
(4-Aza)Trp compared to Trp causes perturbations in the hydrophobic minicore
sufficient enough to interfere with proper folding of the protein. The same is
most likely also true for (7-Aza)Trp. In this case, the combination of repulsive
interactions of the free electron pair in ring position 7 and the increased hydro-
philicity would explain the higher amount of insoluble (7-Aza)Trp-containing
fluorescent proteins compared to (4-Aza)Trp-containing fluorescent proteins, which
only suffer from the changed hydropathy.
Fig. 8 Trp57's local structural environment in ECFP. Surface representation of Trp57 and its
surrounding in ECFP (a). Trp57 has contacts to a number of atoms in its vicinity as depicted in (b).
Ligand bonds are given in
purple
, nonligand bonds in
orange
and hydrogen bonds in
green
(including distances). Nonligand residues involved in hydrophobic contacts are depicted as
red
semicircles
and atoms involved in hydrophobic contacts are depicted as
black circles
. It is worth to
note that from these analyses it seems that there is more spatial freedom at position 4 than at
position 7 for substituent accommodation of the indole side chain [
59
]
Search WWH ::

Custom Search