Environmental Engineering Reference
In-Depth Information
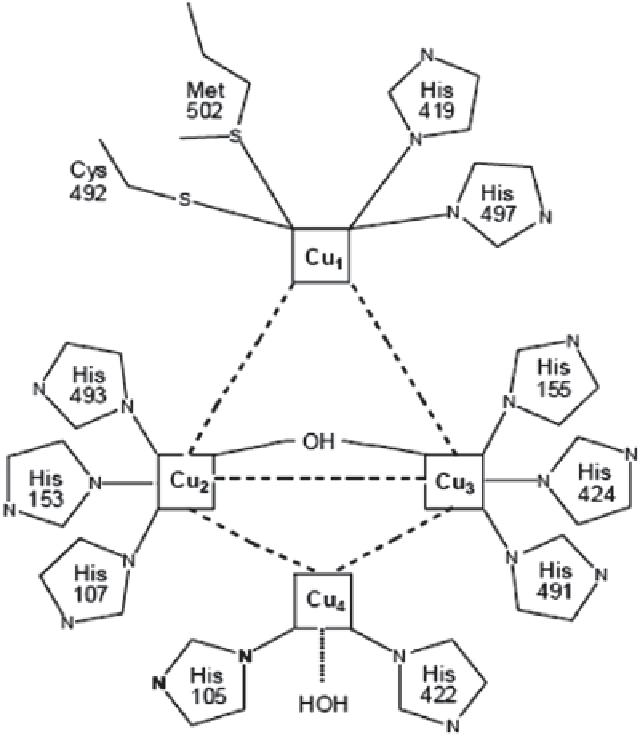
Fig. 2.4
Copper centers of
the laccase. (Adapted from
Claus
2004
)
groups, e.g., with respect to litter mineralization
(Dedeyan et al.
2000
), dye detoxification, and
decolorization (Abadulla et al.
2000
; Kaushik
and Thakur
2013
). Laccases in both free and
immobilized form as well as in organic solvents
have found various biotechnological applica-
tions such as analytical tools—biosensors for
phenols, development of oxygen cathodes in
biofuel cells, organic synthesis, immunoassays
labeling, delignification, demethylation, and
thereby bleaching of craft pulp (Bourbonnais and
Paice
1992
; Bourbonnais et al.
1995
) In addition,
laccases have also shown to be useful for the
removal of toxic compounds through oxidative
enzymatic coupling of the contaminants, lead-
ing to insoluble complex structures (Wang et al.
2002
). Laccase was found to be responsible for
the transformation of 2,4,6-trichlorophenol to
2,6-dichloro-1,4-hydroquinol and 2,6-dichloro-
1,4-benzoquinone (Leontievsky et al.
2000
).
Laccases from white rot fungi have been also
used to oxidize alkenes, carbazole, N-ethyl-
carbazole, fluorene, and dibenzothiophene in
the presence of hydroxybenzotriole (HBT) and
2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic
acid) (ABTS) as mediators (Niku-Paavola and
Viikari
2000
; Bressler et al.
2000
). An isolate of
the fungus
Flavodon flavus
was shown to be able
to decolorize the effluent from a Kraft paper mill
bleach plant.
F. flavus
decolorized several syn-
thetic dyes like azure B, brilliant green, congo
red, crystal violet, and Remazol brilliant blue R
in low nitrogen medium (Raghukumar
2000
).
Partial decolorization of two azo dyes (orange
G and amaranth) and complete decolorization of
two triphenylmethane dyes (bromophenol blue
and malachite green) was achieved by cultures of
Pycnoporus sanguineus
producing laccase as the
Search WWH ::

Custom Search