Environmental Engineering Reference
In-Depth Information
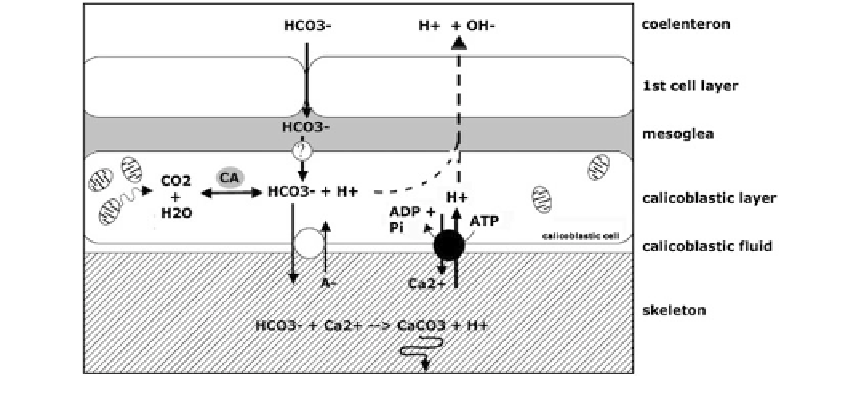
Fig. 6.23
Deposition of calcium carbonate by the outer skin layer or ectoderm at the basal plate of a coral polyp
The bicarbonate ions again diffuse through
the mesoglea, although this process is not yet
completely understood. The next step is, how-
ever, not passive, but active; bicarbonate ions are
pumped into the calicoblastic
Eventually, the bicarbonate and calcium
together precipitate as calcium carbonate
(CaCO
3
). The released protons (H
+
) are con-
stantly pumped back into the coral cells to ensure
a continuous high pH value in the calicoblastic
layer. This has to do with a very important
chemical equilibrium in seawater:
uid by an anti-
porter system which uses up negatively charged
ions (A
−
). Calcium ions (Ca
2+
) are also translo-
cated to the calcifying layer, and H
+
-ions are
pumped into the calicoblastic cells at the same
time. As this process works against a chemical
gradient, it uses up energy, which is provided by
the hydrolysis of ATP to ADP and inorganic
orthophosphate (Pi). Note that most bicarbonates
originate from the coral cell
fl
CO
2
þ
H
2
O
H
2
CO
3
HCO
3
þ
H
þ
CO
2
3
þ
2H
þ
As pH levels drop, more carbonate ions are
converted into bicarbonate ions. This provides
more room for new carbonate ions, such as those
from the coral skeleton. For this reason, corals
maintain high calicoblastic
s own metabolism;
the mitochondria exhale carbon dioxide, after
which the enzyme carbonic anhydrase (CA)
catalyses the reaction to generate bicarbonate
ions (HCO
3
−
). Up to 75 % of the available
bicarbonate originates from the coral itself, and
not from the water column. Eventually, the
bicarbonate and calcium together precipitates as
calcium carbonate (CaCO
3
). The released pro-
tons (H
+
) are constantly pumped back into the
coral cells to ensure a continuous high pH value
in the calicoblastic layer. This remains about 9.3
during the day and drops to about 8 at night.
This means that stony corals grow mostly during
the day.
'
uid pH levels to
prevent newly produced skeleton from redissolv-
ing. The amount of free carbonate ions is called the
aragonite saturation state. During the day, cali-
coblastic
fl
uid pH levels lie around 9.3 and around
8 at night. At these pH levels, calcium carbonate
cannot dissolve properly and precipitates as ara-
gonite. Without this high pH level, coral skeleton
would dissolve quickly. As pH levels drop, more
carbonate ions are converted into bicarbonate
ions. This provides more room for new carbonate
ions to dissolve, such as those from the coral
skeleton. For this reason, corals maintain high
fl