Agriculture Reference
In-Depth Information
ature) can be extracted from thermal denaturation curves, reflecting the global stability of
the folded versus the unfolded protein. 0.4M glycerol,
myo
-Inositol and trehalose increased
the melting temperature of malate dehydrogenase by 3 to 5
o
C as compared to proteins alone
[39].
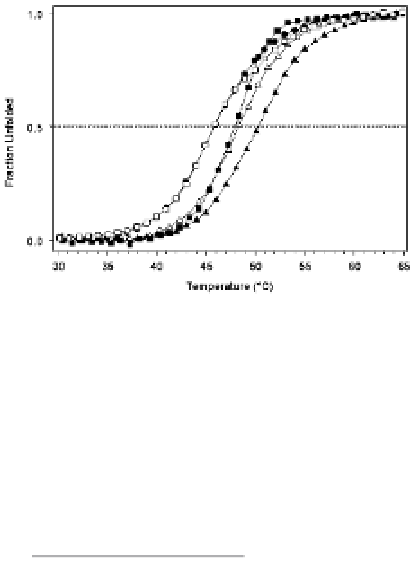
Figure 5.
Thermally induced unfolding of Malate Dehydrogenase (•) in the presence of 0.4M glycerol (□), trehalose
(▵), glucose (▪) and
myo
-Inositol (▴). The horizontal line indicates the midpoint transition temperature (T
m
). Osmolytes
and proteins were mixed to protein solution and equilibrated at room temperature prior to heating. Temperature pro‐
files at 222nm were recorded for 1 °C increments in the temperature range 20-90 °C at a heating rate of 1 °C min
−1
[39]. Thermal unfolding measurements were set up in quartz cuvettes, placed into a Peltier controlled sample holder
unit connected to a temperature probe to provide an accurate temperature record. Thermal unfolding curves were
analyzed using a sigmoidal curve function according to (Equation 1) [40]:
é
ù
(
m T b
´ -
) (
1 ( / )
T
-
m T b
´ -
D
D
N
N
)
q
= ê
+
m T b
´ -
ú
(1)
T
N
N
m
ê
+
T T
ú
ë
û
m
where θ
T
is the ellipticity at temperature T, m
T
is the slope of the curve within the transition
region, and the inflection point of the curve the melting temperature T
m
. At each tempera‐
ture b
N
and b
D
can be extrapolated from the pre- and post-transition baselines, (m
N
× T − b
N
)
and (m
D
× T − b
D
), respectively. The fraction of unfolded protein can be calculated by sub‐
tracting these baselines (Equation 2):
q q
-
q
-
(
m T b
´ -
)
T
N
T
N
N
f
=
=
(2)
v
q q
-
(
m T b
´ -
) (
-
m T b
´ -
)
v
N
D
D
N
N
The stabilization of protein global folds through naturally occurring osmolytes seems to be a
general mechanism. Other studies also reported increases in the midpoint transition temper‐
ature (ΔT
m
) of 2 to 18
o
C upon the addition of 0.1-2M glycerol, trehalose and sucrose meas‐
ured on various proteins [41-43]. Additionally, all proteins studied in the presence of
osmolytes showed a remarkably retention of secondary structure at T
m
relative to proteins
Search WWH ::

Custom Search