what-when-how
In Depth Tutorials and Information
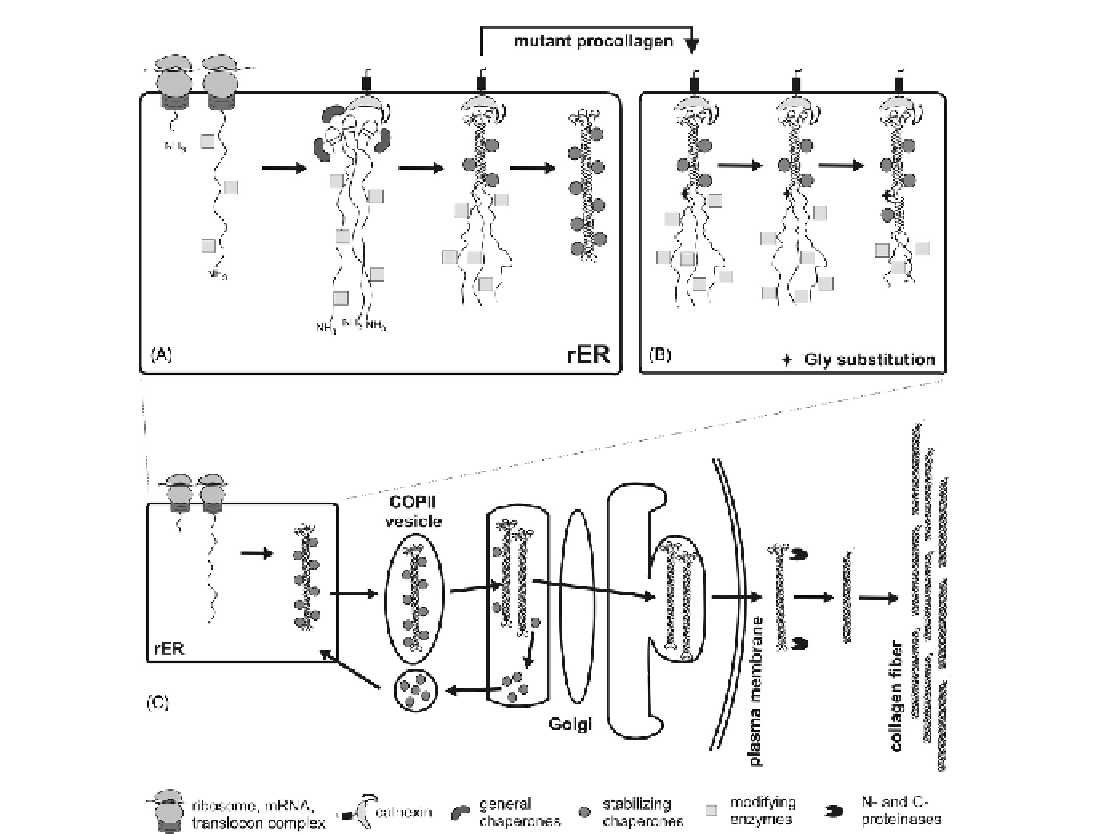
FIGURE 7.1
Simplified scheme of type I collagen biosynthesis. (A) C-propeptide folding is assisted by general ER chaperones, including BiP,
GRP94, PDI, calreticulin and calnexin; calnexin likely anchors procollagen molecules to the ER membrane. Subsequent folding requires stabili-
zation of the forming triple helix by specialized chaperones (HSP47 and potentially other molecules). (B) Gly substitutions and other mutations
that reduce the triple-helix stability (and therefore propensity to triple helix formation) result in a folding delay, until triple-helix formation is
renucleated on the N-terminal side of the mutation. (C) Folded procollagen is transported from the ER to an ER-Golgi intermediate compart-
ment (ERGIC) in large COPII-covered vesicles together with bound HSP47. After dissociation from procollagen in ERGIC
/
Golgi, HSP47 is trans-
ported back to the ER. Procollagen is secreted outside the cell. Its N- and C-propeptides are removed by specialized N- and C-proteinases. This
cleavage, which may begin already in the secretory pathway inside the cell, triggers assembly of collagen molecules into fibers.
COLLAGEN TRIPLE HELIX
structure of mammalian collagens is stabilized pri-
marily by 4-hydroxyproline (4-Hyp) and Arg in the
Y-position as well as by Pro in the X-position.
36,38,39
This
stabilization is essential for procollagen folding; e.g.,
deficient hydroxylation of Y-position Pro into 4-Hyp
by collagen proline 4-hydroxylases in the absence of
ascorbic acid prevents normal synthesis and folding of
procollagen.
40,41
Other X and Y amino acids affect the
triple-helix stability as well, but to a lesser degree.
42
Regions with few or no stabilizing X and Y residues are
generally less stable, more flexible, may exhibit local
unfolding and refolding, and are more susceptible to
cleavage by general proteases.
43-45
Such flexible regions
The distinguishing feature of all collagens is a triple
helix formed by three polypeptide chains with a glycine
residue in every third position. The obligatory glycines
are essential for the triple-helix formation. Gly substi-
tution for another amino acid places the side chain of
the latter inside the helix core, preventing interchain
hydrogen bonding, sterically disrupting the helix and
decreasing the helix stability.
36,37
X- and Y-positions in Gly-X-Y triplets are occupied
by different amino acids that vary along the triple helix.
Studies of model peptides revealed that the triple-helix