what-when-how
In Depth Tutorials and Information
fibrillogenesis;53
53
biglycan and decorin can regulate
growth factor presentation to cellular receptors;
54
and
hyaluronan “captures space” for matrix deposition.
55
found in both α
1
and α
2
procollagen chains with a more
severe phenotype associated with reduced type I colla-
gen synthesis and secretion and deposition of structur-
ally abnormal collagen molecules into the matrix.
62-64
MUTATIONS IN OI
Autosomal Recessive Mutations
Approximately 10% of individuals with OI do not
have mutations in the type I collagen genes. A number
of genes encoding proteins involved in collagen pro-
cessing have been identified as causative factors for an
autosomal recessive form of OI when mutated (
Figure
5.3
). The prolyl 3-hydroxylase complex can function as a
prolyl 3-hydroxylase, a peptidylprolyl isomerase, and a
molecular chaperone.
65
The complex consists of cartilage-
associated protein (CRTAP encoded by
CRTAP
), pro-
lyl 3-hydroxylase 1 (P3H1, encoded by
LEPRE1
) and
cyclophilin B (CYPB, encoded by
PPIB
).
66
Mutations have
been identified in CRTAP,
66-69
LEPRE1
70,71
and PPIB
72,73
causing cases of autosomal recessive OI. Mutations in
another procollagen chaperoning complex encoded by
FKBP10
(FK506 binding protein 65)
74-79
and
SERPINH1
(heat shock protein 47)
80
also cause recessive OI.
Mutations in the telopeptide lysyl hydroxylase encoded
by PLOD2 also associate with recessive OI.
81
A muta-
tion in BMP1, the carboxy terminal propeptidase, also
associates with recessive OI.
81
Two novel mutations in
genes encoding proteins not directly involved in collagen
Autosomal Dominant Mutations
The original clinical classification of OI defined four
types on the basis of phenotypic severity: type I OI has a
mild phenotype of variable skeletal fragility; type II has
a phenotype of extreme osseous fragility, still-birth or
neonatal death; type III has a phenotype of moderate to
severe osseous fragility; and type IV has a phenotype of
moderate osseous fragility.
56
This clinical heterogeneity
was reflected by the variety of mutations in the COL1A1
and COLlA2 genes that underlie most cases of OI.
57
Type
I OI is characterized as having reduced levels (~50%) of
normal collagen triple helical content being secreted by
a cell as a consequence of a null allele that results in pre-
mature chain termination and zero transcription from the
affected allele.
58,59
The lack of secretion of abnormal col-
lagen minimizes the deleterious effects of the mutation.
60
Mutations mapping to carboxy-terminal domains inter-
fering with C-terminal binding and winding up of the
triple helix have been observed in probands with type
II OI.
61
In type III and IV OI patients, defects have been
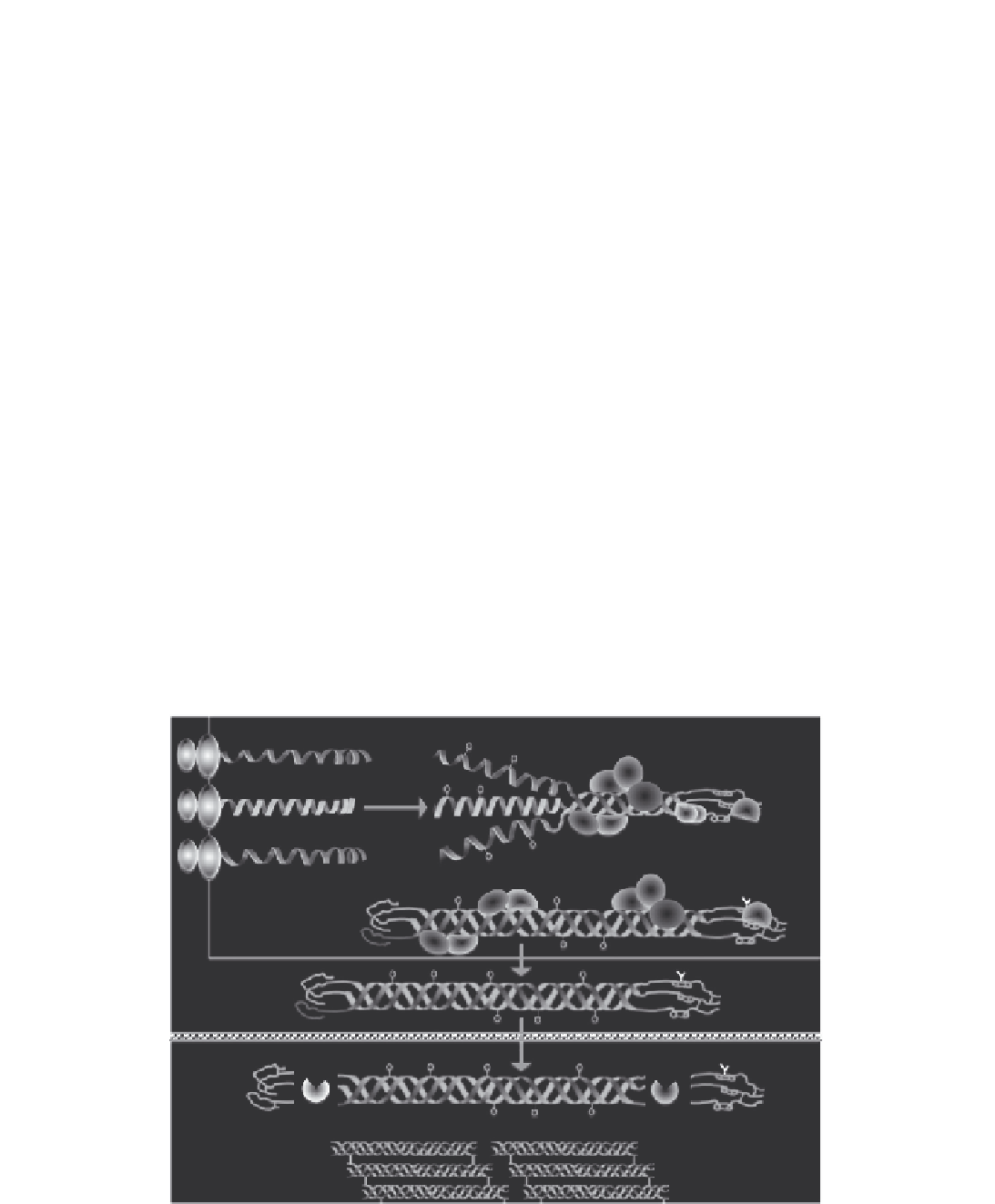
Rough endoplasmic reticulum/golgi
COL1
α
1
CYPB
P3H1
CRTAP
COL1
α
2
PLOD2
COL1
α
1
FKBP65
HSP47
Extracellular matrix
PINP
PICP
BMP1
FIGURE 5.3
Collagen metabolism-related genes mutated in OI. A majority of the mutations that give rise to autosomal dominant OI are
traceable to the type I collagen procollagen genes
COLIA1
and
COLIA2
. Additional proteins found to mutate in autosomal recessive forms of
OI include FK506-binding protein 65 (FKBP65), heat shock protein 47 (HSP47), cartilage-associated protein (CRTAP), prolyl 3-hydroxylase 1
(P3H1), cyclophilin B (CYPB), procollagen telopeptide lysyl hydroxylase 2 (PLOD2), and bone morphogenetic protein 1 (BMP1).