what-when-how
In Depth Tutorials and Information
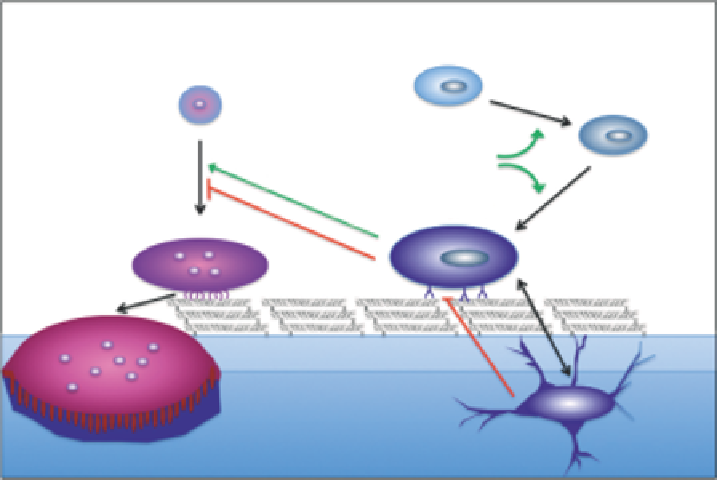
Mesenchymal precursor
Hematopoietic precursor
Pre-osteoblast
WNTs
BMPs
Osteoblast
Pre-osteoclast
Osteoclast
Osteocyte
FIGURE 5.1
Bone cell lineage. Osteoclasts arise from hematopoietic precursor cells and osteoblasts arise from mesenchymal precursor cells.
Osteocytes are differentiated osteoblasts that have become encased within the mineral phase. The transcription factors RUNX2 and OSX play
central roles in osteoblast differentiation. Macrophage colony stimulating factor (M-CSF), receptor activator of NFκB ligand (RANKL), tumor
necrosis factor-α (TNF-α), osteoprotegerin (OPG) and low-density lipoprotein receptor-related protein 5 (LRP5) are produced by cells in the
osteoblastic lineage and regulate bone formation and resorption.
function.
9
Hedgehog, WNT and bone morphogenetic
protein (BMP) signaling pathways promote osteoblast
differentiation, while Notch signaling pathways inhibit
differentiation.
10-14
Osteoblast differentiation is also
modulated by alpha2beta1 integrin binding to type I col-
lagen.
15
A subset of mature osteoblasts becomes embed-
ded in the osteoid matrix that is later mineralized and
these cells become differentiated osteocytes.
16
Osteocytes
have both local and systemic effects thorough sensing
mechanical load, producing sclerostin (SOST, a mol-
ecule that regulates osteoblast function) and FGF-23 (a
phosphatonin that modulates the kidney and phosphate
metabolism).
16-19
The low-density lipoprotein receptor-
related protein 5 (LRP5) acts in osteocytes and per-
haps some late-stage osteoblasts to modulate bone mass
through canonical Wnt signaling.
20,21
formation.
22,23
Integrin-mediated preosteoclast interactions
with type I collagen are also involved in osteoclastogenesis
and remodeling.
24-26
Additionally, the osteoclast-associ-
ated receptor (OSCAR) is a collagen receptor specifically
expressed by preosteoclasts that stimulates osteoclasto-
genesis.
24,27
Thus, there is crosstalk between osteoblasts-
secreted products and osteoclasts which facilitate the
coupling of bone formation and resorption.
Collagen
Type I collagen is the major protein component of
the bone extracellular matrix, accounting for up to 90%
of the organic matrix. Type I collagen synthesis fol-
lows translation in the endoplasmic reticulum of the
pro-collagen alpha-1 (COL1α1) and alpha-2 (COL1α2)
chains (
Figure 5.2
). Nascent collagen chains undergo
a number of post-translational modifications includ-
ing proline and lysine residue hydroxylation, as well
as glucose and galactose attachment to hydroxylysine
residues. Transient association between molecular chap-
erones and pro-collagen chain domains promotes wind-
ing of the triple helix from the carboxy terminal toward
the amino terminal. After secretion, the noncollagenous
propeptides are removed by procollagen amino and car-
boxy proteinases. Processed collagen molecules generally
associate in a parallel but staggered fashion, giving rise
to fibrils with a banding pattern that can be observed by
electron microscopy. Post-translational modifications and
the binding of other matrix components such as small
Osteoclasts
Osteoclasts are enlarged multinucleated cells belong-
ing to a monocyte/macrophage lineage that have spe-
cialized adaptations that enable them to degrade bone.
Osteoclast differentiation involves interactions between
monocytic precursors, osteoblasts, osteoblast-produced
proteins and systemic factors (
Figure 5.1
). Osteoblasts can
secrete proteins that stimulate osteoclast formation such
as macrophage colony stimulating factor (M-CSF), recep-
tor activator of NF-κB ligand (RANKL) and tumor necrosis
factor-α (TNF-α); or osteoprotegerin (OPG), a decoy recep-
tor that blocks RANKL activity and inhibits osteoclast