what-when-how
In Depth Tutorials and Information
4.4
4.2
y = 1.92 + 0.74x
SEE = 0.37
r = 0.40
p < 0.0005
y = 1.02 + 0.98x
SEE = 0.18
r = 0.93
p < 0.0005
y = 0.97 + 1.12x
SEE = 0.24
r = 0.71
p < 0.0005
3.2
4.2
4.0
3.0
4.0
3.8
2.8
3.8
2.6
3.6
3.6
2.4
3.4
2.2
3.4
3.2
2.0
3.2
3.0
1.8
3.0
2.8
1.6
2.8
2.6
1.4
2.6
2.4
1.2
2.4
2.2
1.4
1.6
1.8
2.0
2.2
2.4
1.4
1.6 1.8 2.0 2.2
Body surface area (m
2
)
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
Body surface area (m
2
)
Body surface area (m
2
)
Children/Adolescents
Adults <40
Adults >40
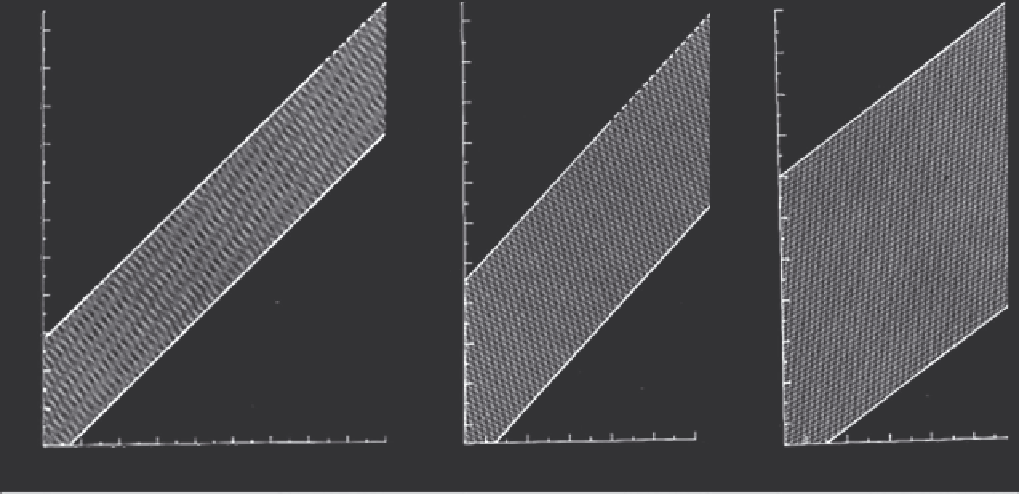
FIGURE 34.1
Aortic root growth curves normalized to body size and age. The left graph is for children and adolescents, the middle is for
adults less than 40 years of age and the right is for adults greater than 40 years of age. The shaded areas represent the range of values in which
95% of the population will fall.
(Reprinted from
16
,
with permission from Elsevier.)
this was also more prominent after controlling for BSA.
3
Univariate and multivariate analyses showed that LV
size and wall thickness correlated with blood pressure,
which was higher in the cohort with OI than in con-
trols. Accordingly, further analysis was performed after
excluding OI patients with hypertension (
N
= 37), and
after indexing for BSA, the wall thickness and LV mass
were still higher in the OI group than controls;
P
<0.05.
3
The collagen content in the extracellular matrix
of wild-type mouse hearts consists of approximately
85% type I collagen.
19
Detailed analysis of the
oim
mouse hearts with mutation in
Col1a2
found that the
hearts in these mice are very similar to controls, based
on heart weight/body weight ratios and echocardio-
graphic assessment of ejection fraction.
20
There is a
mild increase in the LV wall thickness in the
oim
mice,
similar to the mild increase in people with OI noted by
Radunovic and colleagues.
3,20
In addition, there was
less collagen in the
oim
hearts compared to controls, as
measured by collagen fiber analysis and hydroxypro-
line content.
20
In response to cardiac injury, such as acute myo-
cardial infarction, there is normally an increase in the
production of ventricular collagens, including type I.
21
Studies of myocardial infarction in mice with
Col1a2
mutation (
oim
mice) showed that homozygous mutant
mice have increased mortality due to ventricular rup-
ture, compared to heterozygous and wild-type mice.
22
This is associated with less type I collagen prior to
injury, increased production of matrix metallopro-
teinases and early LV dilation.
22
In a different murine
model, experimental myocardial infarction was per-
formed in mice with a mutation in
Col1a1
that substi-
tutes three amino acids at a collagenase cleavage site,
rendering proα1(I) collagen resistant to proteolytic
cleavage.
23,24
These mice have normal myocardial col-
lagen content at baseline and after infarction, but the
cleavage-resistant collagen is associated with LV dila-
tion, diminished cardiac performance and myocardial
fibril organization.
24
These murine studies suggest that
individuals with OI may have impaired response to car-
diac injury.
Right-sided heart failure also occurs in OI, typically
due to pulmonary disease. Several factors contribute to
the pulmonary complications of OI, including scolio-
sis, rib fractures and intrinsic pulmonary parenchymal
disease.
2,25
Increases in the pulmonary arterial pressure
initially cause right ventricular hypertrophy, and this
later causes dilation and dysfunction of the right ven-
tricle. Thiele and colleagues recently investigated the
cardiac and pulmonary findings in mice with
Col1a1
mutation.
26
In addition to a lower left ventricular ejec-
tion fraction in the severely affected subgroup, they
also have right ventricular hypertrophy. These mice
had pulmonary hemorrhage, hypoxia and hypercapnia.
These investigators also studied a cohort of 46 individu-
als with type III and IV OI. The majority (36/46, 78%)
had scoliosis with diminished forced vital capacity.
26