what-when-how
In Depth Tutorials and Information
FIGURE 20.3
Predicted structure of the mutant BRIL/IFITM5. The five amino acids that would be added by the c.-14C>T mutation are
marked in red. TM: transmembrane domain; IC: intracellular domain.
At the functional level, overexpression and knockdown
studies conducted in cultured osteoblasts suggested that
BRIL is a positive modulator of mineralization.
10
Indeed
overexpression of
Bril
in UMR106 osteosarcoma cells pro-
moted mineralization. Conversely, small hairpin RNA
interference of
Bril
expression in MC3T3 abrogated miner-
alization (
Figure 20.4
). The molecular mechanisms of BRIL
action in osteoblasts, however, have not been thoroughly
investigated. Whether BRIL contributes directly to min-
eralization by interacting with its extracellular environ-
ment/matrix and/or indirectly in association with other
membrane and intracellular mediators, is still unknown.
In this respect, BRIL was found through immunoprecipi-
tation experiments to directly interact with other trans-
membrane proteins such as FK506 binding protein 11
(FKBP11), an association that appears to further modulate
other complex assembly with tetraspanin proteins CD9
and CD81.
24
It remains unclear whether those interactions
occur
in vivo
and contribute to BRIL function.
Studies exploring the function of BRIL by genetic
approaches in mice have yielded equivocal evidences.
The
Bril
-speciic global knockout apparently had dif-
ficulty breeding, and displayed only a subtle and tran-
sient reduction in bone length and structure in embryos
and neonatal mice - adults were normal.
25
Notable in
that study was that all bone morphometric parameters
remained unchanged in the knockout. In contrast, our
own
Bril
-speciic knockout mouse did not present any
developmental and reproductive problems, and did not
show any appreciable mineralization defects in their
skeleton (Moffatt, unpublished). In addition, genetic
ablation in mice of either
Ifitm3
alone, or the entire locus
comprising
Ifitm1,
,
2
,
3
, and
Bril
, did not present any
apparent physiological phenotype under normal condi-
tions,
26
and no appreciable skeletal defects (Moffatt and
Thomas, unpublished). Whether there could be any com-
pensation
in vivo
by the other members (IFITM6, 7 and
10) has not been tested so far.
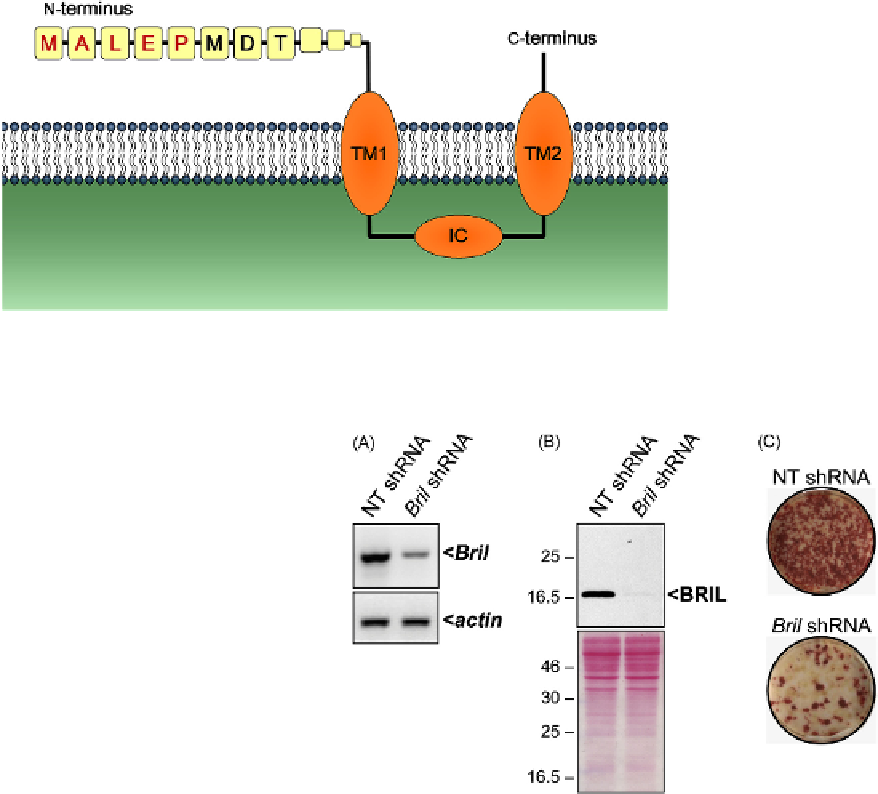
FIGURE 20.4
Knockdown of BRIL in MC3T3 osteoblats affects
mineralization. Stable expression of a non-target- (NT) or a
Bril
-speciic
shRNA in MC3T3 osteoblasts was achieved with lentiviruses. As com-
pared to the control, a significant reduction in
Bril
expression was
observed by RT-PCR (A) and by Western blot analyses (B). The bot-
tom panel in (B) is the corresponding ponceau red staining of the blot.
Representative whole well Alizarin red staining (C) assessed after 15
days in culture indicates that the loss of BRIL causes decreased mineral
deposition.
Clearly the information gained from existing mouse
models has not allowed one to conclusively infer a func-
tion for BRIL in the skeleton, despite marked effects
observed
in vitro
on osteoblast activity. It is even more
difficult to interpret the clinical manifestations observed
in OI type V given our current, somewhat limited,
molecular understanding of BRIL function. At this stage,
we can only speculate as to the effect caused by this rela-
tively simple and apparently innocuous extension at the
N-termini of BRIL. One hypothetical role would be for
mutant BRIL to somehow be involved in differentially
affecting osteoblastic function, depending on the micro-
environment, negatively impeding on trabecular forma-
tion, and promoting bone formation, spontaneously or