what-when-how
In Depth Tutorials and Information
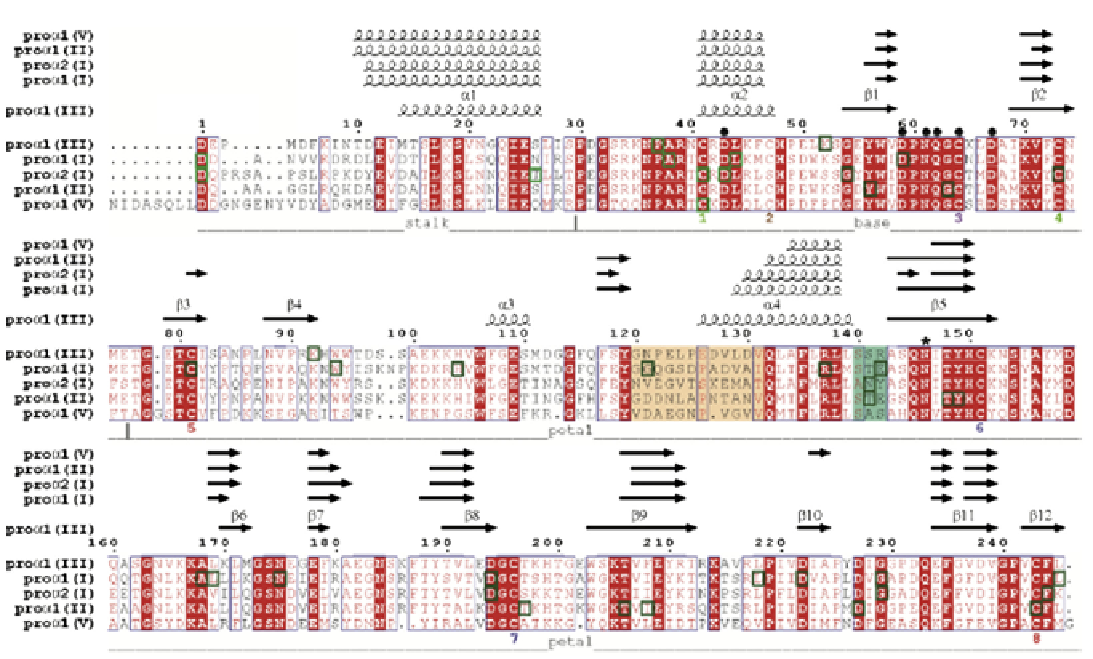
FIGURE 13.3
Alignment of the pro-α1(I), pro-α2(I), pro-α1(II), pro-α1(III) and pro-α1(V) C-propeptides showing the locations of all reported
naturally occurring missense mutations (green boxes; see
Table 13.1
for the
COL1A1
mutations and
Table 13.2
for the
COL1A2
mutations).
Different regions and secondary structure elements found in the procollagen III C-propeptide are also indicated, as are predicted secondary
structures (obtained using PsiPred53) for the other C-propeptides. Also shown are the positions of Cys residues (numbered according to the
sequence and also as Cys 1 to 8) with intrachain disulfide bonds identified as color-matched pairs. Residues involved in Ca2+ coordination
are indicated by
●
and the single N-linked glycosylation site by * (note Asn146 was mutated to Gln in the structure presented here). The long
(12 residue) and short (3 residue) stretches of the discontinuous 15 residue chain recognition sequence are highlighted in wheat and deep
teal color, respectively. Numbering refers to the C-propeptides of the pro-α1(III) chain. Sequence alignments and rendering were done using
CLUSTALW34 and ESPript35, respectively.
(Reprinted from
8
, with permission.)
Pro-
α
2(I) C-Propeptide Mutations
The milder phenotype in patients with alterations
in the pro-α2(I) C-propeptide domain likely reflects
two observations. First, pro-α1(I) chains can substitute
for pro-α2(I) chains in type I procollagen molecules,
as shown by the formation of pro-α1(I) homotrimers,
which are compatible with normal skeletal develop-
ment and function, and thus can compensate for the
loss of the pro-α2(I) chains (although this may be asso-
ciated with EDS features), whereas complete absence of
pro-α1(I) chains is not compatible with life. Second, the
pro-α2(I) collagen chains constitute only one of three
subunits in the fully assembled procollagen trimer,
whereas the pro-α1(I) chain occupies two of the three
positions. This means that, in the case of a pro-α1(I) col-
lagen defect, three-quarters of all possible trimers will
have one or two defective chains, whereas a pro-α2(I)
collagen defect will affect only half of the procollagen
molecules.
63
INVOLVEMENT OF ER-STRESS AND AN
UN
FOLDED PROTEIN RESPON
SE
The ER has a sophisticated quality control system
for ensuring that misfolded proteins do not accumu-
late or follow the secretory pathway, but are instead
retained within the ER and targeted for degradation.
80
This cytoprotective system, known as the “unfolded
protein response” (UPR), has evolved to counter the
stresses that occur in the ER in the presence of misfolded
proteins, but it can also contribute to the pathophysiol-
ogy of many heritable disorders of the ECM.
81,82
Non-
glycosylated proteins that misfold or fold too slowly are
recognized through interactions with chaperones such
as BiP, and are targeted for degradation.
83
Degradation
of misfolded proteins can (at least) be performed by
two mechanisms. First, the protein can be retrotranslo-
cated back into the cytoplasm where it is ubiquinated
and degraded by the proteasome in a process known