as well as chimeras that contained only prM and E from JE.
GENE THERAPY
Chimeras containing C, prM, and E from JE were not viable,
whereas chimeras containing only prM and E from JE were
A number of genetic diseases result from the failure to pro-
viable and grew well in culture (Fig. 11.9).
duce a specific protein, usually due to a single defective gene.
The viable chimeras were first tested in mice. The
One of the more exciting possible uses for virus vectors is for
chimera containing the Nakayama strain proteins caused
the expression of a missing protein as a cure for the genetic
lethal encephalitis in mice, as does the YF 17D virus (even
defect associated with its absence. Some of these "monogenic
though it is safe for use in humans). However, the chi-
diseases" that might be curable through the use of gene therapy
mera containing prM and E from the attenuated JE strain
are listed in Table 11.1. For successful treatment, expression
was fully attenuated in mice and did not cause illness. The
of the missing protein must be long-term and preferably
fully attenuated chimera was chosen for testing in mon-
lifelong, the levels of protein produced must be sufficient to
keys, and was found to be safe and to protect monkeys
alleviate the symptoms of the disease, the protein must be
against challenge with JE virus.
expressed in or translocated to those cells that require the nor-
Clinical trials of this candidate vaccine have taken place in
mal protein for function, and infection with the virus vector
humans. The vaccine appears to be safe and more effective than
must be free of disease symptoms. Because of the requirement
the JE vaccines now in use. Furthermore, this approach is appli-
for long-term expression, viruses whose DNA integrates into
cable to other flaviviruses, such as the dengue viruses, for which
the host chromosome, such as the simple retroviruses as well
no licensed vaccines exist, or West Nile virus, which spread
as the lentiviruses, and adeno-associated viruses, offer the most
recently to the Americas where it caused a number of fatal cases
promising system for many diseases. To date, several hundred
of human encephalitis. Recombinant YF 17D expressing the
patients have been treated with vectors based on Moloney
prM and E proteins of all four serotypes of dengue viruses and
murine leukemia virus in clinical trials. Clinical trials have also
recombinant viruses expressing prM and E of West Nile virus
been conducted that use adenovirus. adeno-associated virus,
are also in clinical trials with encouraging results.
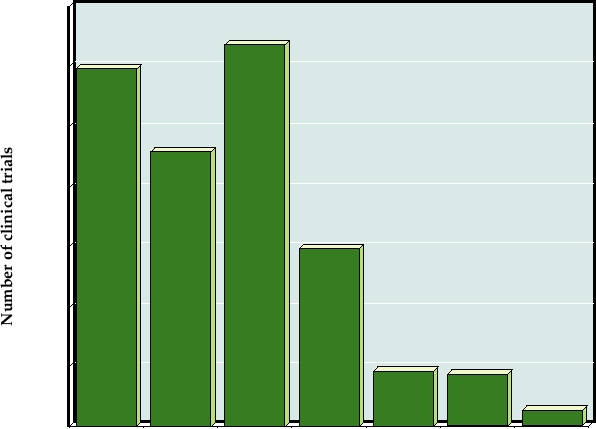
poxvirus, and herpesvirus vectors (Fig. 11.10).
Yet another possible approach to developing new gen-
Clinical trials in humans, which require extensive prior
erations of vaccines using the power of biotechnology is to
testing in animals, are divided into three phases. Phase I
attenuate a virus by making changes in the laboratory that
involves relatively few, usually healthy individuals. The
are expected to cripple the virus. Such an approach can be
objective of Phase I trials is to test the safety of a vaccine or
used with virtually any virus. A candidate vaccine strain of
treatment as well as the dosage that is tolerated, and the indi-
dengue virus has been constructed by making deletions in
viduals are closely monitored during the trial. Phase II trials
the 3′ nontranslated region of the genome that attenuate the
involve more individuals and test the efficacy of the treat-
virus, and such viruses are being tested in early trials.
ment, and patients are again closely monitored. If a treatment
TABLE 11.1
Genetic Defects That Are Candidates for Gene Therapy
Disease
Defect
Incidence
Target cells
Severe combined
Adenosine deaminase (ADA)
Rare
Bone-marrow cells or T lymphocytes
immunodeficiency (SCID)
in 25% of SCID patients
A
Factor VII deficiency
1:10,000 males
Liver, muscle, fibroblasts or bone
Hemophilia
B
Factor IX deficiency
1:30,000 males
marrow cells
Familial hypercholesterolemia
Deficiency of low-density
1:1 million
Liver
lipoprotein (LDL) receptor
Cystic fibrosis
Faulty transport of salt in lung epithelium
1:3000 Caucasians
Airways in the lungs
(Structural) defects in the α or
Hemoglobinopathies
1:600 in certain
Bone marrow cells that are precursors
β globin gene
thalassemias
ethnic groups
to red blood cells
Gaucher's disease
Defect in the enzyme glucocerebrosidase
1:450 in Ashkenazi Jews
Bone marrow cells, macrophages
α1 antitrypsin deficiency
Lack of α1 antitrypsin
1:3500
Lung or liver cells
inherited emphysema
Duchenne muscular dystrophy
Lack of dystrophin
1:3500 males
Muscle cells
Xeroderma pigmentosa
Impaired DNA repair, leading to
Rare
Fibroblasts
severe sensitivity to sunlight
350
300
250
200
150
100
50
0
Adenoviral
Poxviral
AAV
Herpesviral
Plasmid or
Retroviral,
Others
naked
including
DNA
lentiviral
Mode of Gene Delivery
FIGURE 11.10 Vectors used in gene therapy trials as of January 2007. The "others" category includes flavivirus (5),
measles virus (3), Newcastle disease virus (1), poliovirus (1), Semliki Forest virus (1), Sendai virus (1) and SV40 (1).
Data are from Gene Therapy Clinical Trials Worldwide Web site of the Journal of Gene Medicine at: http://www.wiley.
co.uk/genetherapy/clinical/.
passes both of these tests, Phase III trials can begin in which
antigens. Often only the gene of interest and the viral tran-
thousands of individuals are treated to test the efficacy of
scriptional regulatory elements are left, and to prepare the
treatment. Virtually all of the clinical trials in gene therapy
vectors for use in trials, all other functions must be supplied
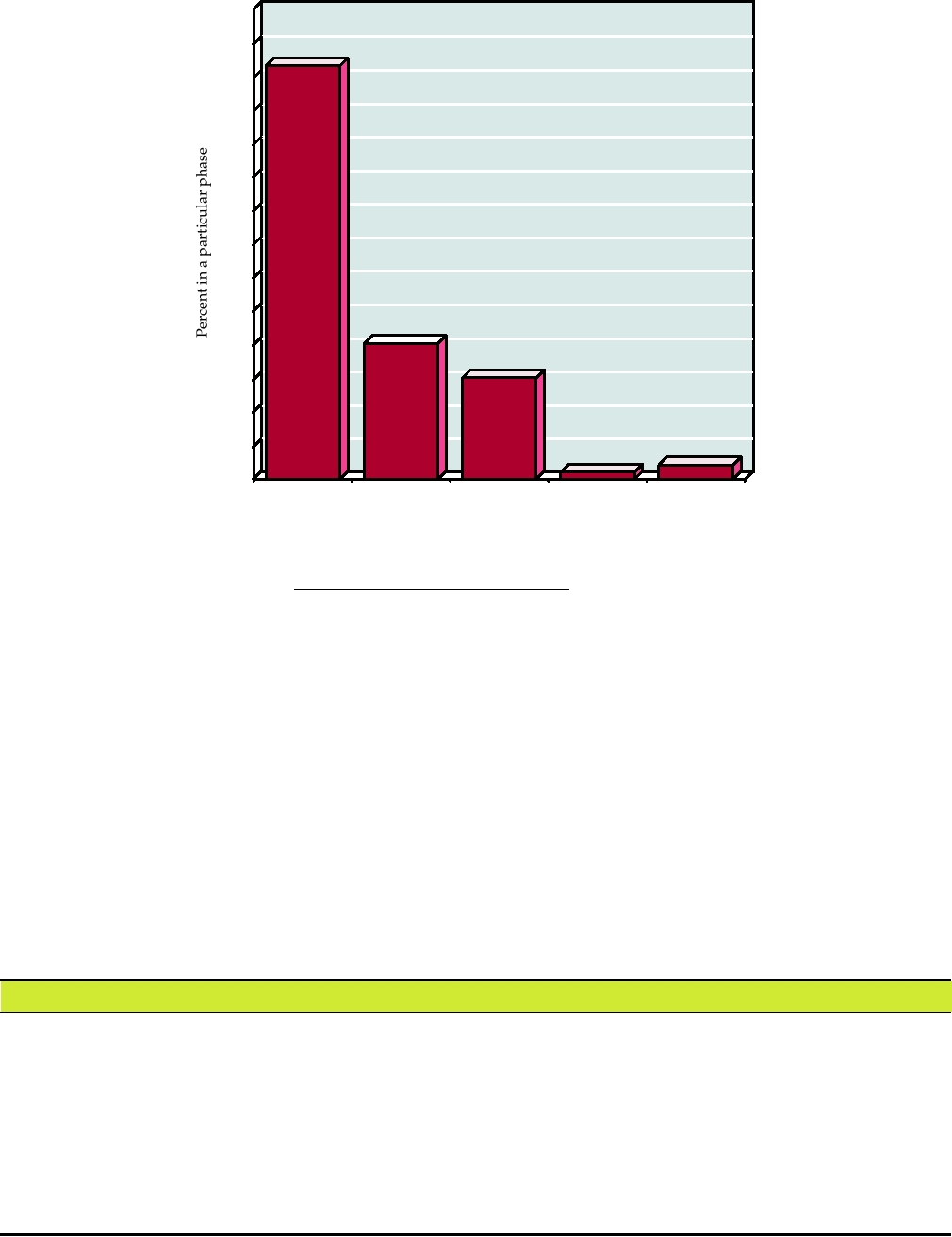
conducted to date are Phase I or Phase II; fewer than 2% of
by a helper virus or a packaging cell line. Another advantage
trials have progressed to Phase III (Fig. 11.11), and no gene
of such stripped down vectors is the fact that it is improbable
therapy treatments have been licensed to date.
that the vector can recombine with wild-type viruses, either
In addition to the possible treatment of genetic defects,
exogenous or endogenous, to cause disease.
virus vectors may also be useful for the treatment of a number
A partial listing of clinical trials that attempt to treat sev-
of acquired diseases. These include cancer, HIV infection,
eral different genetic defects by using virus vectors to deliver
Parkinson's disease, injuries to the spinal cord, and vascular
specific genes is given in Table 11.5. There have been few
diseases such as restenosis and arteriosclerosis. A partial listing
successes to date and the table is more of a compendium of
of acquired diseases that have been suggested as candidates for
the variety of genes and diseases, as well as the variety of
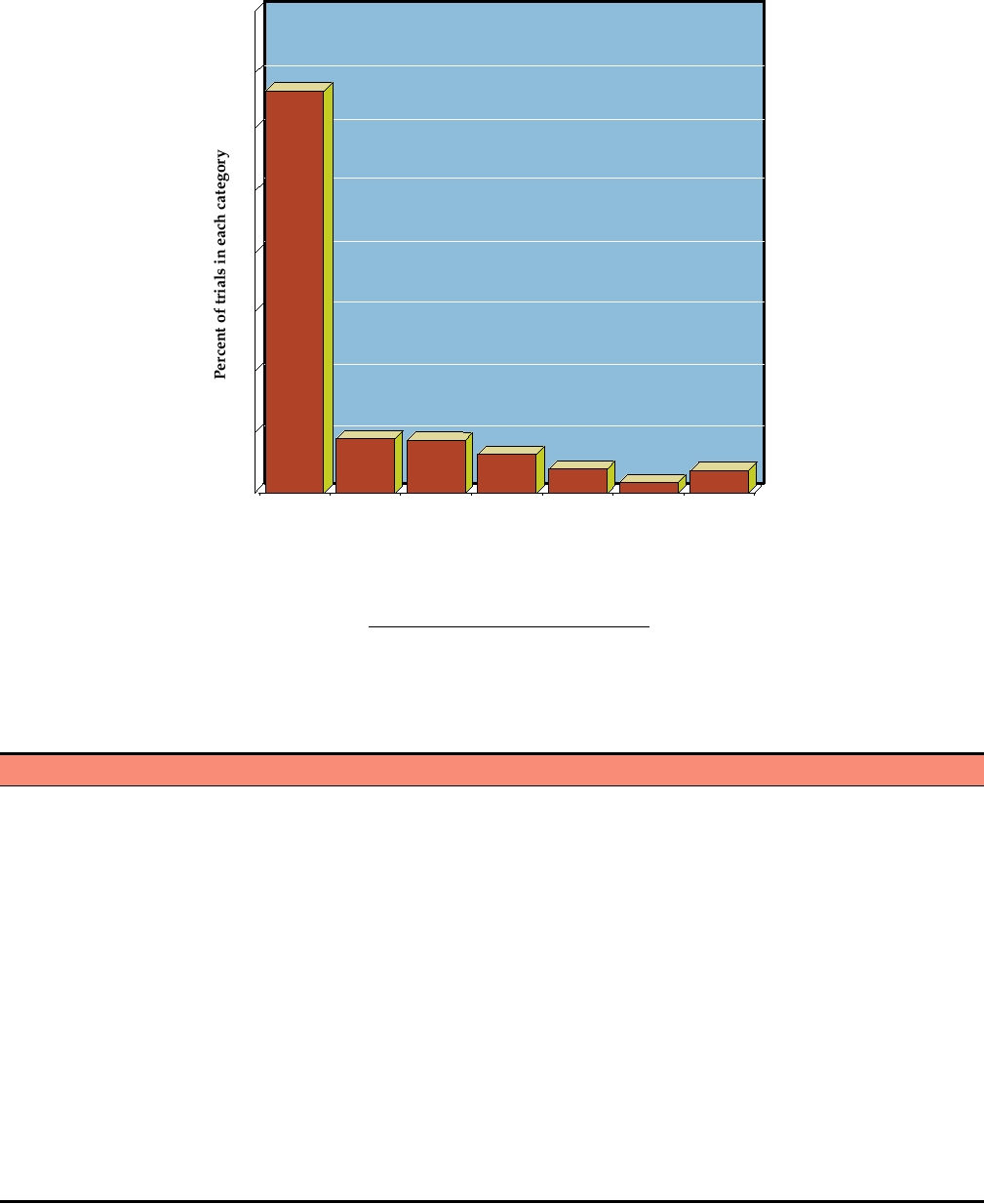
gene therapy is given in Table 11.2, and the number of trials for
delivery schemes, that are being examined. Also included in
a number of different conditions is shown in Fig. 11.12. Despite
the table is an impending trial for the vaccination of humans
the large efforts to use gene therapy in clinical settings, the
against HPIV-3 using a bovine virus.
progress has been disappointingly slow, and many of the trials
have been aborted due to unforeseen adverse consequences.
Retrovirus Vectors to Genetically Mark Cells
Nevertheless, as infectious clones of viruses continue to
be developed, a large body of research is being devoted to
Retroviruses have been used in a number of clinical
construction of vectors, especially now to second and third
trials to genetically tag cells. Although this use does not fall
generation vectors, as the problems associated with the initial
within the narrow definition of gene therapy, it does provide
systems are becoming clear. A comparison of various virus
background experience in the use of retrovirus vectors in
systems that are being considered for gene therapy is shown
humans. One such use has been in bone marrow transplanta-
in Tables 11.3 and 11.4. Naked DNA has also been used in
tion for leukemia. Severe forms of leukemia can sometimes
a recent trial for coronary artery disease, and the proper-
be treated by ablation of the hematopoietic system with
ties of this system are included in Table 11.4. Most of the
chemotherapy and/or X-rays in order to kill all tumor cells,
modern vectors have had more and more of the dispensable
followed by reconstitution of the system by transplantation
viral genes deleted. Deletion of these genes reduces patho-
of bone marrow from a compatible donor. Although often
genicity, and prevents the production of immunogenic viral
successful, the leukemia sometimes recurs and it is desirable
70
(775)
60
50
40
30
(255)
20
(190)
10
(27)
(13)
0
I
I/II
II
II/III
III
Clinical phase of trial
FIGURE 11.11 Percent of gene therapy clinical trials that are in each phase as of January 2007. The numbers above
the bars are the actual number of trials. Data from Gene Therapy Clinical Trials Worldwide from the Web site of the
Journal of Gene Medicine at: http://www.wiley.co.uk/genetherapy/clinical/.
to know whether it recurs because of incomplete destruc-
Adenosine is toxic at high concentrations, producing a variety
tion of the patient's leukemic cells or whether the donor cells
of symptoms. The most serious symptom results from the
are the source of the leukemia. Experiments in which the
extreme sensitivity of T cells to elevated adenosine concentra-
donor cells have been tagged using retroviruses that express
tions. Loss of T cells results in SCID, severe combined immu-
a marker gene have been used to answer this question, which
nodeficiency. Both CTL responses (which are T-cell based) and
is important for the design of transplantation protocols.
humoral responses (which require T-helper cells) are impaired.
People with SCID syndrome are unable to mount an immuno-
logic response to infectious agents, and SCID is invariably
Gene Therapy for ADA Deficiency
fatal early in life unless treated in some way. ADA deficiency
Patients who lack the enzyme adenosine deaminase (ADA)
accounts for about 25% of SCID syndromes in humans.
will die early in life unless treated. Lack of ADA results in
SCID can be treated by bone marrow transplantation if
the failure to clear adenosine from the body and, consequently,
a suitable donor can be found. In the case of SCID due to
the accumulation of adenosine in cells throughout the body.
ADA deficiency, weekly or twice weekly injections of ADA
TABLE 11.2
Some Acquired Diseases That Are Candidates for Gene Therapy
Disease
Defect
Incidence
Target cells
Cancer
Many causes, including genetic
1 million/year in
Variety of cancer cell types, in liver,
and environmental
United States
brain, pancreas, breast, kidney
Neurological diseases
Parkinson's, Alzheimer's
1 million Parkinson's and
Neurons, glial cells, Schwann cells
spinal-cord injury
4 million Alzheimer's
patients in the United States
Cardiovascular
Restenosis, arteriosclerosis
13 million in United States
Arteries, vascular endothelial walls
Infectious diseases
AIDS, hepatitis B
Increasing numbers
T cells, liver, macrophages
Rheumatoid arthritis
Autoimmune inflammation of joints
Increasing numbers with
Intra-auricular delivery and expression
of IL-1 and TNF-α inhibitors
aging population
80
(842)
60
40
20
(113)
(106)
(81)
(50)
(47)
(21)
0
Healthy
Infectious
Vascular
Others
Gene
Monogenic
Cancer
volunteers
diseases
diseases
marking
diseases
Conditions currently addressed by gene therapy clinical trials
FIGURE 11.12
Percent of gene therapy trials which are directed toward various conditions as of January 2007. The
numbers above the bars are the actual number of trials. Data from Gene Therapy Clinical Trials Worldwide from the Web
site of the Journal of Gene Medicine at: http://www.wiley.co.uk/genetherapy/clinical/.
TABLE 11.3 Comparison of Properties of Various Vector Systems Based on RNA Viruses
and Retroviruses
Negavirala
Features
Simple retroviral
Lentiviral
Alphaviral
Coronaviral
~4 kbb
Maximum insert size
77.5 kb
77.5 kb
5 kb
2.7 kb
>108
>108
>109
>108
>109 for VSV, PIVs
Concentration in viral
particles/ml
101000 fold lower
Route of gene delivery
Ex vivo
Ex/in vivo
In vivo
In vivo
In vivo
Integration
Yes
Yes
No
No
No
Duration of expression in vivo
Shorter than theorized
Long
Short
Variable
Not known
Stability
Good
Not tested
Good
Dependent on
Good
background
Ease of preparation scale up
Pilot scale up to
Not known
Not known
Not known
Not known
2050 liters
Preexisting host immunity
Unlikely
Unlikely, except in
No
Unlikely
Unlikely
AIDS patients
Safety problems
Insertional mutagenesis?
Insertional
Few
Recombination
--
mutagenesis?
with wild strains
Other advantages
--
Replicate in
--
--
Recombination virtually
nondividing
unknown; naturally
cells
atttenuated viruses exist
a
Includes consideration of VSV based and PIV based vectors.
b
Longer inserts are tolerated but vectors are too attenuated in vivo to be useful.
Source: Verma and Somia (1997), Jolly (1994), and Bukreyev et al. (2006).
TABLE 11.4
Comparison of Properties of Various Vector Systems Based on DNA Viruses
Vectors based on DNA viruses
Naked/lipid
Features
Adenoviral
AAV
Herpesviral
Vaccinia
Baculovirus
DNA
Maximum insert size
7.5 kb
<4 kb
~30 kb
2575 kb
>38 kb
Unlimited size
>1010
>1012
>1010
107109
>108
No limitation
Concentration in viral
particles/ml
Route of gene delivery
Ex/in vivo
Ex/in vivo
Ex vivo
Ex/in vivo
Ex/in vivo
Ex/in vivo
Integration
No
Yes/no
No
No
No
Very poor
Duration of expression
Short
Long
Short/long in CNS?
Short
Short
Short
in vivo
Stability
Good
Good
Unknown
Good
Good
Very good
Ease of preparation
Easy to scale up
Difficult to purify,
Not yet tried
Vaccine
Easy to
Easy to scale up
scale up
difficult to
production
scale up
scale up
facilities exist
Immunologic
Extensive
Not known
Not known
Extensive
Not known
None
problems
Preexisting host
Yes
Yes
Yes
Diminishing as
Unlikely
No
immunity
unvaccinated
population grows
Safety problems
Inflammatory
Inflammatory
Neurovirulence?
Disseminated vaccinia
None?
None?
response,
response,
Insertional
in immuno-
toxicity
toxicity
mutagenesis
compromised hosts
Source: Verma and Weitzman (2005), Jolly (1994), Boulaiz et al. (2005), Hu (2006).
mixed with polyethylene glycol (PEG) have been used to
treatment was available were treated with improved retro-
successfully treat about 60 patients in whom bone marrow
viral therapy. Both patients developed functional immune
transplantation cannot be used because of the lack of com-
systems and no adverse events have been reported. The
patible donors. Of these, about 10 patients have also been
improved results appear to arise from improved protocols
treated with retroviral vectors that express ADA. In these
as well as to selection in the patient for lymphoid progenitor
experiments, T cells were taken from the patient (or in the
cells that expressed adequate amounts of ADA.
case of three newborns, umbilical cord cells were used),
infected ex vivo with the retrovirus vector using a number
Treatment for SCID Caused by IL2RG Deficiency
of different cell culture and infection protocols, and the cells
reinfused into the patient. Many of the patients continue to
SCID disease can also be caused by a failure to produce
produce ADA from the vector several years after treatment.
the receptor for the cytokine interleukin-2 (Chapter 10).
However, all of the patients continue to receive ADAPEG
Thirteen SCID patients with this deficiency were treated in
injections, which is known to be an effective treatment.
two different clinical trials with retroviruses that expressed
Although some patients who have received retroviral ther-
the defective gene (Table 11.5). The results illustrate the
apy have been partially weaned from the supplementary
highs and the lows of gene therapy trials. All 13 patients
ADAPEG, it appears that some of these, and perhaps all,
developed functional immune systems, and the trials at first
do not produce enough ADA to be cured. Thus, although
appeared to be a complete success. However, three of the
no cures were effected in these early trials, the results were
patients later developed T-cell leukemia and one has died
encouraging and suggested that future protocols might be
of the leukemia. The leukemia was at first suspected to arise
more successful. Two areas of retroviral therapy that needed
from insertional mutagenesis, a chronic worry with vectors
improvement were to increase the efficiency with which
that insert into the host DNA, and many gene therapy trials
stem cells are infected, and the need to prevent the retroviral
that used retroviral vectors were suspended. Recent studies
promoter from being downregulated.
indicate that the disease is not due to insertional mutagen-
A more recent trial involving two SCID-ADA patients in
esis, however, but rather due to the oncogenic potential of
Italy was more successful (Table 11.5). Two infants for whom
the IL2RG gene itself, as studies have shown that overex-
no compatible donor existed and for whom no PEG-ADA
pression of this gene in mice results in leukemia.
TABLE 11.5 Recent Human Gene Therapy Trials
Therapeutic
Total
Vector/
Disease
gene
patients
promoter
Method of delivery
Outcome
Monogenic diseases
OTC deficiency
OTC cDNA
18
E1E4 deleted
In vivo injection to
No clinical benefit; 1 death
adenovirus/CMV
hepatic artery
Factor IX deficiency
Modified Factor
8
rAAV2/ CMV
In vivo intramuscular
No sustained clinical benefit
(hemophilia B)
IX gene
7
rAAV2/APOE-
In vivo injection to
Transient Factor IX expression, no
SERPINA1
hepatic artery
sustained benefit
SCID-X1
IL2RG cDNA
10
Retrovirus/MLV-
Ex vivo transduction of
9 patients developed functional
CD34+ cells
(French trial)
LTR
immune system; 3 developed
T-cell leukemia, 1 death
SCID-X1
IL2RG cDNA
4
GALV-pseudotyped
Ex vivo transduction of
4 patients developed functional
CD34+ cells
(British trial)
retrovirus/MFG-LTR
immune system; no adverse
results reported in first 2 years
SCID-ADA
ADA cDNA
2
Retrovirus/MLV-
Ex vivo transduction of
2 patients developed functional
CD34+ cells
(Italian trial)
LTR
immune system; no adverse
results reported in first 4 years
CGD
GP91PHOX
2
Retrovirus/SFFV-LTR
Ex vivo transduction of
2 patients developed functional
CD34+ cells
neutrophils and clonal myelo-
proliferation (cancer)
Duchenne muscular
Dystrophin
9
Plasmid/CMV
Intramuscular injection
Low dystrophin expression in 6 of
dystrophy
9, no adverse effects (Phase I)
Microdystrophin
?
AAV/CMV
Intramuscular biceps injection
Phase I/II ongoing
2OMeAOs
9
Oligonucleotide
Injection into extensor
Successful non-human primate
digitorum brevis
study; human Phase I/II not yet
opened for patient recruitment
Vaccine for infectious disease
HPIV-3 vaccine
F and HN surface
--
BPIV-3
Infection
Successful non-human primate
glycoproteins
study; human Phase I trial not
yet opened for patient recruitment
APOE-SERPINA1, α1-antitrypsin promoter linked to APOE enhancer; BPIV-3, bovine parainfluenzavirus 3; CGD, chronic granulomatous disease;
CMV, cytomegalovirus promoter; GALV, gibbon ape leukemia virus; HPIV, human parainfluenzavirus; LTR, long terminal repeat; MFG, derivative
of MLV; MLV, Moloney murine leukemia virus; OTC, ornithine transcarbamylase; rAAV, recombinant adeno-associated virus serotype 2; SCID-
ADA, severe combined immunodeficiency secondary to adenosine deaminase deficiency; SCID-IX, severe combined immunodeficiency secondary to
mutations in the IL2RG gene; SFFV, Friend mink cell spleen focus-forming virus.
Source: Data for this figure came from Porteus et al. (2006), Foster et al. (2006), Bukreyev et al. (2006).
Cystic Fibrosis
mucus that is a characteristic of cystic fibrosis. In addition,
the expression needs to continue for the life of the patient,
Cystic fibrosis results from loss of the cystic fibrosis
which means either a very stable (integrated?) gene being
transmembrane conductance regulator (CFTR), which regu-
expressed, or a system of repeated administration of vector
lates epithelial transport of ions and water. Although lack of
that can be tolerated without immunologic consequences.
this protein results in damage to the epithelium in many parts
Lentiviruses have been proposed as an attractive vector, but
of the body, the most serious manifestation is lung disease
there have been concerns about probable pathogenesis due
accompanied by chronic bacterial infection of the airways.
to the lentivirus itself. However, for this disease, the most
Clinical trials using adenoviral vectors, which infect respi-
promising mode of gene delivery so far developed has been
ratory epithelium, to express CFTR in the lungs have been
DNA compacted into nanoparticles with polycations, in par-
conducted. The first such studies were encouraging, but a
ticular PEG-polylysine, nicknamed "polyplexes." A clini-
more recent trial that was carefully controlled found no relief
cal trial in humans used CFTR polyplexes, and the Phase I
of symptoms. Inflammation produced by the high doses of
trial showed no adverse effects. For this disease a nonviral
adenovirus used in trials is also a problem. It is difficult to
approach may well be the best solution.
get efficient delivery to the lung, especially through the thick
Duchenne Muscular Dystrophy
a frameshift mutation in one of the exons, such that no dys-
trophin is produced. Since severely truncated dystrophin (like
Duchenne muscular dystrophy (DMD) is a severe mus-
micro-dystrophin) can be functional (Fig. 11.13), therapy to
cle wasting disorder due to the lack of functional dystrophin
get rid of the exon causing the problem is being developed.
protein. It occurs in 1/3500 male births. There are a number
Modified antisense oligonucleotides (AOs) can be used to
of difficulties in attempting to cure DMD with gene therapy,
alter the splicing pattern of the gene such that the exon in
including the size of the protein, which is encoded in a cDNA
which the frameshift occurs is skipped, thereby restoring
of 11kb, and the need to deliver the vector to a large propor-
the reading frame. Following success of an AO with a
tion of the body mass, that is to all of the striated muscles and
2′O-methyl-phosphorothiolate backbone (2OMe AOs) to
cardiac muscle. Several approaches have been tried, and the
restore function in the mdx mouse, two clinical Phase I trials
first human clinical Phase I trial has just been completed (see
have been initiated, one in the Netherlands using 2OMe AOs
Table 11.5). In this study, a plasmid containing the entire gene
and one in Great Britain using morpholino AOs. Since both
under the control of a CMV promoter was injected intramus-
are targeting the exclusion of exon 51, it will be possible to
cularly. Although only low levels of dystrophin expression
directly compare the two chemically modified AOs.
were observed in 6 out of 9 patients, there was no evidence
for adverse reactions and the trial was considered a success.
A second approach has been to attempt to introduce the
Rheumatoid Arthritis
gene in an AAV vector, especially in one of the many human
isolates to which most of the population show no preexisting
Rheumatoid arthritis is a chronic, progressive inflamma-
immunity, or into a nonhuman AAV. However, here the size
tory disease of the joints. An estimated 5 million people in
of the gene is a problem, but it has been shown that the
the United States suffer from it. There is no cure. Drug thera-
dystrophin protein contains a large number of repeated ele-
pies are used that ameliorate the symptoms, but most of these
ments (Fig. 11.13) and that "mini-dystrophin" and "micro-
drugs have side effects and cannot be taken indefinitely. If
dystrophin" are functionally active in the mouse model for the
the disease progresses far enough, joint replacement may
disease, the mdx mouse. Low-pressure intravenous injection of
be required. The disease is associated with the release of
AAV6 expressing micro-dystrophin into a mouse could trans-
inflammatory cytokines in the affected joints. Clinical trials
fect 90% of muscle, but high titers of the AAV6 vector were
have started that use retroviruses to deliver the gene for an
required. A Phase I/II clinical trial has been initiated for deliv-
anti-arthritic cytokine gene to the joints. The gene encodes
ery of micro-dystrophin in AAV under the control of a CMV
the interleukin (IL)-1 receptor antagonist, which inhibits the
biological actions of both IL-1α and IL-1β. It is hoped that
promoter into human patients, but there are no results as yet.
A third approach attacks the specific nature of the genetic
such treatment might damp out the disease or at least keep it
defect. It has been shown that 75% of DMD is caused by
from progressing.
cDNA 11 kb
CR
CT
H1 1 2 3 H2 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 H3 20 21 22 23 24 H4
A
cDNA 6.3 kb
CR
CT
H1 1 2 3 19 H3 20 21 22 23 24 H4
B
Cysteine-rich domains
Carboxy terminal domains
cDNA 3.8 kb
CR
1 2 19 H320 21 22 23 24 H4
Actin binding domains
Hinge regions
C
Spectrin repeats
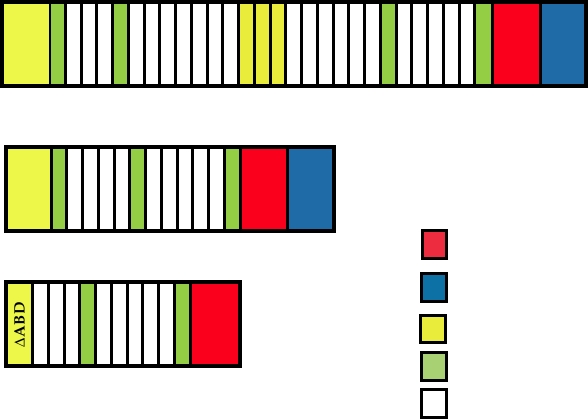
FIGURE 11.13 Forms of the dystrophin protein. (A) Full-length dystrophin containing all functional domains.
(B) Mini-dystrophin from a Duchenne muscular dystrophy patient who was only mildly impaired. (C) Micro-dystrophin
engineered for delivery in AAV vectors. Adapted from Figure 1 in Foster et al. (2006).
A Gene Therapy Failure
I/II, but more than 1000 patients were participating in the
trials listed in the table. As shown in Fig. 11.12, the devel-
Patients who have deficiencies in enzymes that participate
opment of gene therapy approaches for the control of can-
in the urea cycle have increased concentrations of ammonia
cers continues to attract much effort, such that the majority
in the blood. High concentrations of ammonia result in vari-
of clinical trails to date have been directed against cancers.
ous symptoms, which can include behavioral disturbances
Although progress has been painfully slow and disappoint-
or coma. Severe deficiencies in these enzymes result in
ing, the prospect remains that effective treatment may yet be
early death, but moderate deficiencies can result in delayed
obtained for at least some cancers using such approaches.
appearance of symptoms and may be partially controlled by
In most of the trials in Table 11.6, viruses are used to
diet. One such enzyme is ornithine transcarbamylase (OTC),
express proteins that control the growth of tumors or that
which is found on the X chromosome. Deficiencies in OTC
are toxic to tumor cells. A number of different cytokines
are therefore more common in males than in females.
are being tried as antitumor agents, such as IFN-γ, IL-2,
Gene therapy trials that use virus vectors recently received
TNF, and GM-CSF. Another approach is to try to repair the
a major setback when a relatively fit 18-year-old male with
defective regulatory gene in the tumor cell, which is often
an inherited deficiency for OTC died 4 days after an ade-
p53. Many other gene products are also being tested. The
novirus vector was injected into his liver. A high dose of
viruses used to express these products include the retrovi-
adenovirus (4 × 1010) that expressed OTC was injected in an
ruses, the adenoviruses, or the poxviruses. More recent tri-
effort to achieve adequate levels of enzyme production. The
als have used additional viruses as well, in particular the
virus unexpectedly spread widely and a systemic inflam-
herpesviruses and the adeno-associated viruses. Herpes
matory response developed, inducing a fever of 40.3°C. He
simplex type 1 would seem to be particularly appropriate
went into a coma, his lungs filled with fluid, and he died of
for control of brain tumors, because the virus is neurotropic
asphyxiation. This unfortunate result makes clear the pos-
but sets up a latent infection in neurons. One idea would
sible drawbacks to experimental treatments and the difficul-
be to engineer herpes to express a protein that is only toxic
ties in designing protocols that allow an adequate margin of
in dividing cells but which would be nontoxic in mature,
safety while trying to achieve a clinically relevant result.
nondividing neurons. The table also lists a number of trials
that use lipofection to introduce the gene of interest into
target cells.
Treatment of Restenosis
Further afield, thought is being given to the possibility of
A gene therapy trial in patients with heart disease gave
using viruses to express proteins that are overexpressed in
very encouraging results. Although this study did not
tumor cells in an attempt to stimulate the immune system to
involve virus vectors, a brief description will be given since
respond by killing tumor cells. This is in essence an attempt
it serves as an incentive for continuation of gene therapy
to vaccinate a person against a tumor. For this approach to
trials. Coronary artery disease is common in older people.
succeed, an antigen overproduced by a tumor cell, such as
Angioplasty or bypass surgery is used to open clogged arter-
a melanoma cell, must be identified, inserted into a suitable
ies, but in many patients the arteries close up again (a process
vector, and the person with the tumor infected with the virus
called restenosis). Thirteen patients with chronic chest pain
vector in an attempt to stimulate the immune system. In prin-
who had failed angioplasty or bypass surgery or both were
ciple, this approach may be feasible, but only time will tell
injected in the heart muscle with DNA encoding vascular
whether it is in fact practical.
endothelial growth factor. This factor promotes the growth
Another approach is to try to direct the virus, more or
of blood vessels, a process called angiogenesis. Two months
less specifically, to infect the tumor cells, so that upon
after treatment, all patients exhibited an improvement in
infection the cells are killed. Cell death might result either
vascularization of damaged areas of the heart, as shown
because the virus itself is cytolytic or because the virus
by imaging and mapping studies. All patients reported a
expresses a protein that renders the cell sensitive to a toxic
decrease in disease symptoms, and all had an improved per-
agent such as BUdR. A number of the trials listed in Table
formance in treadmill tests. Although the number of patients
11.6 use the TK gene for this, since cells that express TK
is small, the uniformly positive results are encouraging.
are sensitive to BUdR. One possible approach is to engineer
the virus so that its surface glycoprotein expresses a
monoclonal antibody directed against an antigen expressed
Viruses as Anticancer Agents
only on the tumor cell, while at the same time causing the
virus to be unable to infect cells that do not express the
A large number of clinical trials have examined the pos-
antigen. Experiments have established the possibility of
sibility of using viruses as anticancer agents. Table 11.6 lists
this approach, at least in principle, with viruses such as
a number of trials that were active in the year 2000, to give
the alphaviruses. Another approach was illustrated by the
a flavor of what is being tried and the number of maligna-
experiments with VSV to design a virus that could infect
cies that are being considered as candidates for treatment
only HIV-infected cells.
using gene therapy approaches. These trials are all Phase I or
TABLE 11.6
Clinical Trials of Gene Transfers for Cancer Therapy in the United States in 2000
Genea
Phaseb
Location
Vector
Number of trials
Number of patients
Brain cancers
IFNγ
Neuroblastoma
Retrovirus
1
4
I
IL-2
Retrovirus
1
12
I
IL-2
Adenovirus
1
6
I
Central nervous system
TK
Adenovirus
2
22
I
Pediatric tumor
TK
Retroviral producing cells
1
2
I
Adult brain tumor
TK
Retroviral producing cells
1
15
I
Ovarian cancer
HSV-TK
Adenovirus
1
10
I
TK
Retroviral producing cells
3
42
I
BRCA-1
Retrovirus
1
40
I/II
p53
Adenovirus
1
16
I
Small cell lung cancer
IL-2+NeoR
Lipofection
1
8
I
Anti-sense to k-ras
Retrovirus
1
9
I
p53
Adenovirus
2
59
I/II
Prostate cancer
GM-CSF
Retrovirus
1
8
I/II
PSA
Poxvirus
1
3
I
HSV-TK
Adenovirus
1
18
I
Breast cancer
BRCA-1
Retrovirus
1
21
I
E1A
Lipofection
1
16
I
MDR-1+NeoR
Retrovirus
4
39
I
CD80
Lipofection
1
15
I
CEA
Poxvirus
4
53
I
CEA
RNA transfer
1
30
I
Melanoma
GM-CSF
Gene gun
1
17
I
GM-CSF
Retrovirus
2
29
I
HLA-B7/β2 m
Lipofection
8
165
I/II
IL-2+NeoR
Retrovirus
5
115
I
IFNγ
Retrovirus
3
91
I
TNF+NeoR
Retrovirus
1
12
I/II
MART-1
Adenovirus
1
33
I
MART-1
Poxvirus
2
16
I
gp100
Poxvirus
1
19
I
gp100
Adenovirus
1
7
I
CD80
Lipofection
1
17
I
Miscellaneous carcinomas
p53
Adenovirus
1
26
I
HLA-B7/β2m
Lipofection
4
76
II
IL-2
Lipofection
1
11
I
CEA
Poxvirus
1
8
I
Lymphomas and solid tumors
IL-2
Retrovirus
2
29
I
TK
Retrovirus
1
11
I
IL-12+NeoR
Retrovirus
1
31
I
Bladder cancer
p53
Adenovirus
1
5
I
Colo/rectal, renal, and liver cancers
GM-CSF
Retrovirus
1
18
I
HLA-B7/β2m
Lipofection
4
53
I/II
CD
Adenovirus
1
6
I
IL-4
Retrovirus
1
18
I
TNF+NeoR
Retrovirus
1
12
I
(Continued)
Search WWH :