characterized by mild influenza-like symptoms only and one
the African/Southeast Asian region at some time in the dis-
fatal case characterized by pneumonia followed by respira-
tant past. These different dengue viruses then jumped inde-
tory distress syndrome. The virus was eradicated by culling
pendently into humans to become human dengue viruses. A
of poultry in the country.
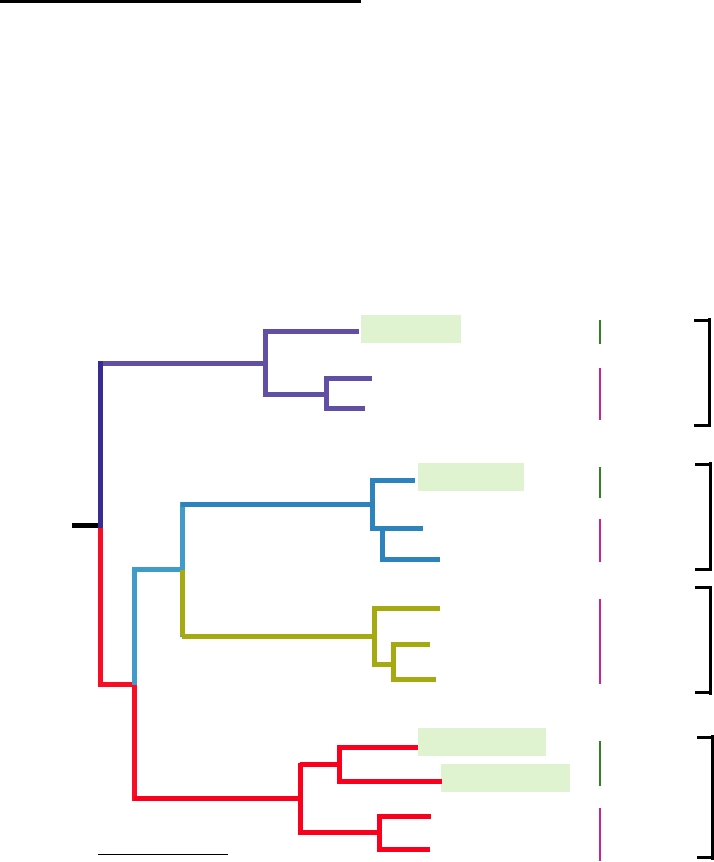
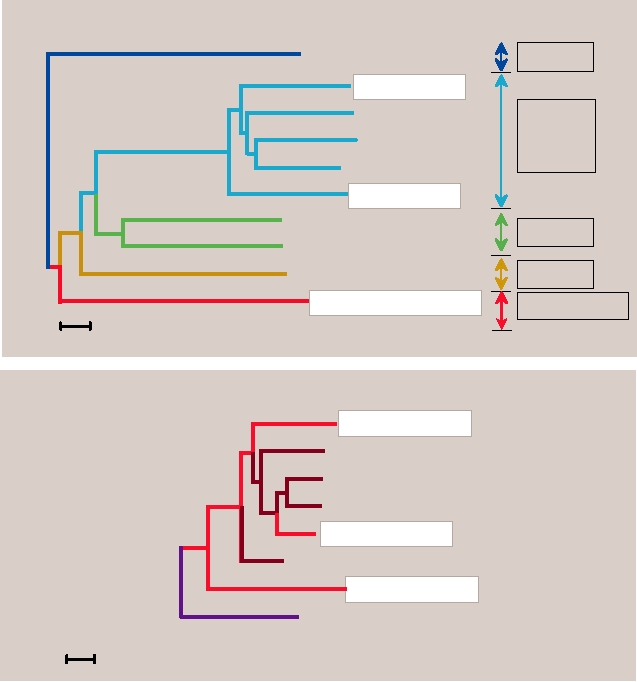
dendrogram of dengue viruses of monkeys (sylvatic strains)
and humans (endemic/epidemic strains) is shown in Fig. 8.7.
Notice, for example, that the monkey dengue-4 virus groups
H9N2 Influenza
with human dengue-4 but forms a distinct lineage from that
In 1999, two cases of human infection by H9N2 influ-
of the other dengue viruses. Similarly, monkey dengue-1 and
enza A occurred in Hong Kong and five cases in Guangdong
dengue-2 group with the respective human viruses but form
Province, China. Then in 2003 another case occurred in Hong
distinct lineages. Sequencing of sylvatic dengue-3 has not
Kong. The disease was characterized by mild influenza-like
yet been done but it will presumably group in the same way.
symptoms and recovery was uneventful.
From the extent of the divergence in sequences between
the monkey viruses and the human viruses, it is estimated
that the jump to humans occurred on the order of 200 years
VIRUSES ASSOCIATED WITH PRIMATES
ago for DEN-1, 600 years ago for DEN-4, and 1000 years
ago for DEN-2. It is further estimated that the African and
Dengue Virus
Malaysian sylvatic viruses diverged about 800 years ago.
Such estimates are subject to considerable uncertainty but
The Origin of Dengue Viruses
are probably valid to within a factor of two. Since human
Dengue viruses are mosquito-borne flaviviruses that
dengue virus is an exclusively human virus that is epidemic
cause widespread epidemics in humans (Chapter 3). Forest
in nature and induces lifelong immunity, it could not have
cycles of dengue have been documented in Africa and
existed until human populations were large enough to sup-
Southeast Asia in which the vertebrate reservoir is mon-
port the continued existence of such a virus. This topic is
keys and various species of Aedes mosquitoes maintain the
covered in more detail in Chapter 4 when discussing measles
virus. Recent sequencing studies have now shown that the
virus, but sufficiently large human populations arose only
sylvatic monkey viruses evolved in monkeys into four sero-
within the last thousand years or so in the regions in which
types. This evolution from a common ancestor occurred in
dengue viruses first arose and flourished.
Sylvatic
Malaysia 75
Dengue-4
Thailand 63
Endemic/
Epidemic
Indonesia 76
Malaysia 72
Sylvatic
Dengue-1
Endemic/
Brazil 90
Epidemic
Philippines 84
Puerto Rico 77
Endemic/
Dengue-3
Sri Lanka 81
Epidemic
Thailand 87
Malaysia 70
Sylvatic
Senegal 70
Dengue-2
Trinidad 53
Endemic/
Epidemic
0.1
Thailand 64
FIGURE 8.7 Phylogenetic tree of the four dengue virus types derived from E protein gene nucleotide sequences of
sylvatic (in monkeys) and representative endemic/epidemic (in humans) DEN strains using maximum parsimony. The
scale shows a genetic distance of 0.1 or 10% nucleotide sequence divergence. Adapted from Wang et al. (2000).
Dengue in the Americas
aegypti from large regions, in order to control viral diseases
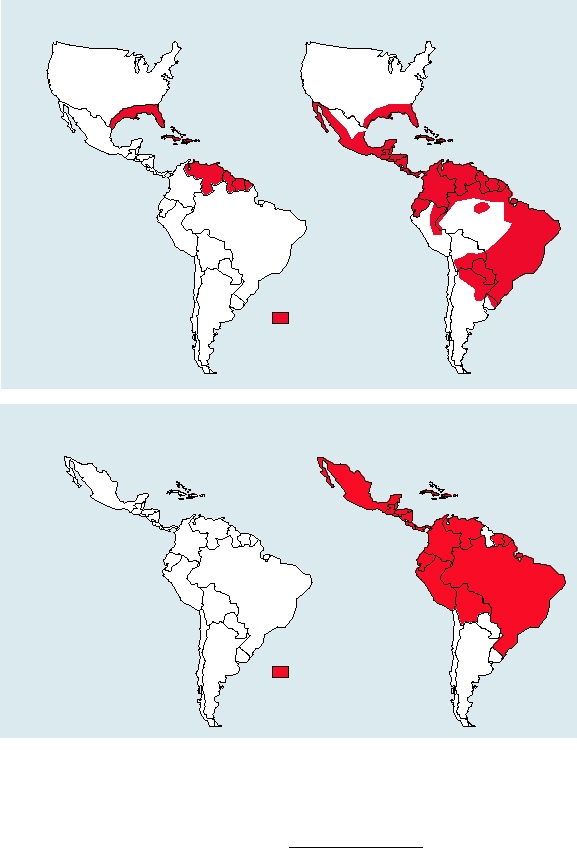
Dengue viruses have been continuously active over large
spread by these mosquitoes, which include not only dengue
areas of Asia and the Pacific region for many years. They
but also yellow fever and other arboviruses. These efforts
have recently dramatically expanded their range in the
succeeded in eliminating the mosquito from large areas
Americas. The viruses may have caused large epidemics in
of Central and South America, as illustrated in Fig. 8.8A.
the Americas, including the United States, in the 1800s and
However, in 1970 these efforts were abandoned because of
into the early 1900s. However, it is impossible to determine
the expense involved and the detrimental effects of DDT on
with certainty from descriptions of the disease written at
the environment, and by 2000 the mosquito had reestablished
the time whether dengue was the causative agent of these
itself over most of the region (Fig. 8.8A). The reintroduction
epidemics or whether other viruses that cause similar ill-
of multiple dengue strains into the Americas from foci in
nesses might have been responsible. In any event, dengue
Asia after the reestablishment of Ae. aegypti resulted in the
almost died out in the Americas following World War II
outbreak of dengue hemorrhagic fever associated with huge
because of efforts to control Aedes aegypti, the urban vector
epidemics of dengue fever (Fig. 8.8B). The history of the increas-
of the virus. With the discovery of DDT in the mid 1900s,
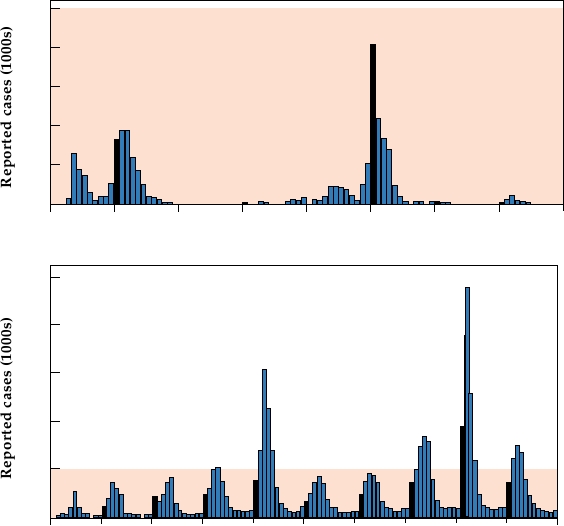
ing infection rate is illustrated in Fig. 8.9 by data from Brazil.
a serious effort was made in the Americas to eradicate Ae.
Before 1993, epidemics of dengue were sporadic, occurring
A
Aedes aegypti in the New World
2000
1970
Range of Aedes aegypti
mosquitos
B
Dengue hemorrhagic fever in the New World
1981-2003
<1981
Countries with laboratory
confirmed dengue
hemorrhagic fever
FIGURE 8.8
Changing distribution of dengue hemorrhagic fever (DHF), and the vector for dengue virus in the New
World. (A) Distribution of the vector mosquito Aedes aegypti in the Americas in 1970 and 2000. Aedes aegypti spread
rapidly during this period due to the collapse of mosquito control programs and urbanization. (B) Increase and spread of
dengue hemorrhagic fever, from 1981 to 2003. Data for these graphs came from the dengue fever information sheets from
the Centers for Disease Control and Prevention Web site at: html://www.cdc.gov.
A
50
40
30
20
10
0
1986
1987
1988
1989
1990
1991
1992
1993
Year
B
250
200
150
100
50
0
1994
1995
1996
1997
1998 1999
2000
2001
2002
2004
Year
FIGURE 8.9
Number of dengue fever cases reported per month in Brazil (A) between 1986 and 1993 and (B) between
1994 and 2004. Note that the areas shaded in pink in the two graphs represent the same number of cases (50,000). Bars
for January cases are filled in black. Adapted from Siquiera et al. (2005).
every few years and then dying out. After this, however,
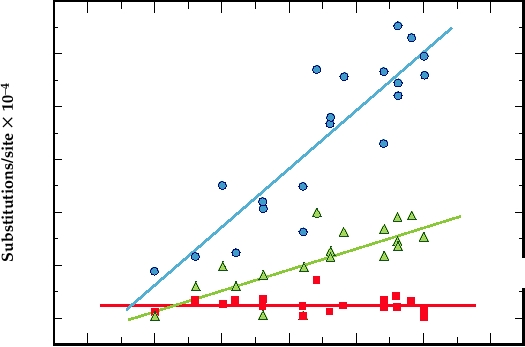
The evolution of DEN-1 in the Americas after its intro-
epidemics have occurred every year and the total number
duction in 1977 is illustrated in Fig. 8.10. Sequencing of
of cases has increased dramatically (notice the difference
strains isolated in various years after 1977 show that silent
in scales).
nucleotide substitutions (i.e., synonymous substitutions that
Prior to the 1980s, a "native American" strain of DEN-
do not result in a coding change) have been fixed at the rate
2 circulated and there was very little dengue hemorrhagic
of 0.2% per year. However, essentially no coding changes
fever (DHF) in the Americas. A strain of DEN-3 circulated
have occurred. Thus, coding changes are not acceptable and
in the 1960s and 1970s but it then disappeared. DEN-1 was
viruses containing such changes do not persist.
introduced into the Americas in 1977 and DEN-4 in 1981
and these viruses then radiated throughout large regions of
Human Immunodeficiency Virus
the Caribbean and northern South America. The first epi-
demic of DHF occurred in 1981, but interestingly, it was
HIVs are human viruses that have become established in
due to the introduction of a new strain of DEN-2 from Asia.
the human population within the last century. The two human
This DEN-2 strain grows more vigorously than the native
viruses HIV-1 and HIV-2 derive from different simian immu-
American strain, which is not associated with DHF, and led
nodeficiency viruses (SIVs), HIV-1 from SIVcpz and HIV-
to the DHF epidemic. Then in 1994 the Southeast Asian
2 from SIVsmm. Further, HIV-1 has become established at
strain of DEN-3 responsible for the DHF epidemic in Sri
least three times by independent entry of SIVcpz into humans.
Lanka described in Chapter 3 reached the Americas. The
A phylogenetic tree of the primate lentiviruses is shown in
result of all these introductions has been a dramatic increase
Fig. 8.11. There are three lineages of HIV-1, all of which
in the incidence of DHF and dengue shock syndrome (DSS)
are related to SIV isolated from chimpanzees (SIVcpz). Of
in the Americas. Whereas in the 1970s there were very few
these three lineages, the M lineage is found worldwide and is
cases of DHF in the Americas, there were more than 10,000
responsible for the majority of human infections. The O line-
cases in the 1980s and more than 60,000 cases in the 1990s.
age is found only in western Africa and in France. The N lin-
Cases have continued to increase in number. In the last 5
eage represents a third introduction of SIVcpz into humans.
years (20012005 inclusive), more than 50,000 cases have
The structure of the dendrogram makes clear that these three
been reported that resulted in about 700 deaths.
viruses independently entered the human population.
600
R = 0.88 (p < 0.0001)
y = 0.0058 + 0.0022t
500
400
300
R = 0.76 (p = 0.00011)
y = 0.00098 + 0.00079t
200
100
R = 0.010 (p = 0.966)
y = 0.0210 + 0.000027t
0
-5
0
5
10
15
20
25
Years
FIGURE 8.10 Relationship between the number of total (▲), synonymous (●), and nonsynonymous (■) nucleotide
substitutions per site and the number of years since the introduction of dengue 1 into the Americas in 1977. R is the
regression constant. Adapted from Figure 2 in Goncalvez et al. (2002).
Epidemiology of SIVcpz
appear to cause disease in chimps, similar to the case for
other SIVs that infect African monkeys.
Until recently, the extent of SIVcpz infection of chimpan-
Surprisingly, SIVcpz is itself a recombinant virus. The
zees was not known. The chimp is an endangered species,
5′ half of the genome is derived from SIV infecting red-
limited in numbers, difficult to study, and only a few isola-
capped mangabeys, whereas the 3′ half is derived from SIV
tions of SIV from chimps had been made. In recent studies
infecting greater spot-nosed or mustached or mona monkeys
to examine the extent of SIV infection of wild chimps, 1300
(Fig. 8.13). The recombination probably occurred in a chimp
stool samples from wild chimps were collected in the field
that had been infected by the two SIVs. Chimps eat other
and laboriously tested for the presence of anti-SIV antibod-
monkeys and could have become infected in this process in
ies and for SIV RNA by RTPCR. The individual responsi-
the same way that humans probably became infected with
ble for the stools was identified by examining the host DNA
SIVcpz upon slaughtering and eating chimps. The fact that
in the sample using highly polymorphic microsatellite loci.
only two of the four subspecies of chimps are infected with
Several different chimp populations were included in these
SIVcpz argues that this virus arose after subspeciation of the
studies.
chimps had taken place. The spread of this virus in chimps
There are four different subspecies of chimpanzee. The
might in fact be a fairly recent occurrence.
type subspecies, Pan troglodytes troglodytes, is found in
West Africa in southern Cameroon, Gabon, and Congo (Fig.
8.12). Pan t. schweinfurthii is further east, primarily in the
Establishment and Spread of HIV
Democratic Republic of Congo but penetrating northward
into the Central African Republic and eastward into a swath
After infection of humans by SIVcpz, the virus had to
from southern Sudan down to Tanzania. Pan t. vellerosus is
adapt to humans and become a human virus in order to be
north of the range of troglodytes, in northern and western
transmitted from person to person and to spread widely.
Cameroon. Finally, Pan t. verus is west of the range of velle-
It seems probable that humans have become infected with
rosus, in a broad zone from Senegal to Ghana. Subspeciation
SIVcpz repeatedly but in most cases the virus failed to adapt
has resulted in part because chimps do not swim and large
to humans or failed to become epidemic because of the low
rivers fragment the various populations. Of these four sub-
transmissibility of the virus. Following a human infection,
species only two, troglodytes and schweinfurthii, are natu-
it may have smoldered in a small number of people but
rally infected by SIV. Rates of infection vary in different
then died out. When the viruses crossed the species barrier
populations but average about 20%, with some populations
and became firmly established in the human population as
exhibiting almost 50% infected individuals. Thus, SIVcpz
HIV-1 is not clear. HIV-1 has been isolated from serum
is a naturally occurring, widespread virus for which two
collected in 1959 in Zaire and antibodies to HIV have
subspecies of chimps are the reservoir. The virus does not
been found in serum collected in 1963 in Burkina Faso, so
SIV syk
SIVsyk
HIV-2 ROD
SIV mac
SIVsmm,
SIVmac,
SIV stm
HIV-2
SIV smm
HIV-2 EOH
SIV mnd
SIVmnd
SIV l'hoest
SIV agm
SIVagm
SIVcpz, HIV-1
SIVcpz, HIV-1
0.02 substitutions per site
HIV-1 group M
SIVcpzGAB2
SIVcpzUS
SIVcpzCAM
HIV-1 group N
SIVcpzGAB1
HIV-1 group O
SIVcpzTAN1
0.10 substitutions per site
FIGURE 8.11 Phylogenetic trees of the primate lentiviruses. Upper panel: a tree constructed using the neighbor-joining
method on selected SIV and HIV pol sequences. Horizontal branch lengths are to the scale shown below. HIV strain names
are arbitrary. SIV names include the name of the host from which they were obtained: syk, Sykes monkey; smm, sooty
mangabey; mac, rhesus macaque; mnd, mandrill; l'hoest, l'hoest monkey; agm, African green monkey; cpz, chimpanzee. The
boxes at the right give the names of the five major lineages of primate lentiviruses identified to date. Redrawn from Whetter
et al. (1999), Figure 1. Lower panel: a tree constructed from maximum-likelihood analysis of full-length env sequences from
HIV-1 isolates of groups M, N, and O and a number of SIV strains from chimpanzees, corresponding to the box on the lowest
branch in panel (A). Human viruses are in red (within a white box), viruses from Pan troglodytes troglodytes are in dark red,
and the strain from P. t. schweinfurthii is in purple. Note the difference in scale. Redrawn from Sharp et al. (2005).
HIV-1 has been in the human population at least that long.
virus in humans, and therefore for the selection of such
From sequencing studies of the glycoprotein gene and
transmissible mutants.
examination of the rate of divergence, one estimate is that
HIV-2 represents a distinct lineage that is closely related
the virus might have entered the human population about
to SIV of sooty mangabey monkeys (smm) and of macaques
70 years ago, although estimates of divergence rates are
(mac). SIV of African green monkeys (agm) and of man-
controversial. Recent changes in human behavior, includ-
drills (mnd) form other lineages that are more closely related
ing more extensive travel by truck, bus, and plane, changes
to HIV-2 than to HIV-1. It is clear that SIVsmm and SIVagm
in sexual practices, and the use of injectable drugs, as well
are naturally occurring infectious agents that are widespread
as the increase in the human population, could have allowed
in Africa and have coevolved with their monkey hosts.
the virus to reach major population centers and spread
Sequence comparisons have shown that different isolates of
more extensively than in the past, becoming epidemic
SIV group with their hosts rather than by geography, and they
worldwide. The spread of the virus could also have been
are therefore adapted to their hosts. They cause no disease
aided by the appearance of mutants that were more easily
in their natural host, but SIVsmm does cause AIDS when
transmissible from person to person. The large increase in
transferred to Asian macaques in captivity. HIV-2 is found
population during the last century has certainly resulted in
primarily in western central Africa, where its distribution
more opportunities for the introduction and spread of the
is almost coincident with that of mangabey monkeys. It
Search WWH :