Viruses That Contain Double-Stranded
RNA: Family Reoviridae
INTRODUCTION
Many of the Reoviridae are transmitted by arthropod vec-
tors (Tables 5.1 and 5.2). The orbiviruses are transmitted
by phlebotomine flies, ticks, gnats, and midges of the genus
Most of the dsRNA-containing viruses that we know about
Culicoides, and members of this genus are true arboviruses,
belong to the family Reoviridae. All members of this large
as are the coltiviruses, transmitted by ticks, and the sea-
family have a genome consisting of 10, 11, or 12 segments
dornaviruses, transmitted by mosquitoes. The members of
of dsRNA totaling 1627 kb. The family is very successful.
the three genera of plant reoviruses are also transmitted by
Twelve genera are currently recognized and different viruses
arthropods and are effectively plant arboviruses (although
infect a wide spectrum of vertebrates, invertebrates, plants, and
the term arbovirus is reserved for vertebrate viruses that are
fungi. Several viruses are important pathogens of humans.
transmitted by arthropods). Thus, members of eight genera
Other dsRNA viruses in addition to the family Reoviridae
of Reoviridae possess the ability to replicate in arthropods,
include the birnaviruses, icosahedral viruses 60 nm in diam-
of which two genera contain viruses that replicate exclu-
eter containing two genome segments of dsRNA totaling
sively in insects and six genera contain viruses that have an
about 7 kb, which infect chickens, fish, and arthropods; the
alternate vertebrate or plant host. The relationships among
totiviruses of fungi and protozoa, which have only one RNA
these various genera are illustrated in the unrooted dendro-
segment; and the partiviruses and hypoviruses of fungi and
gram in Fig. 5.1, which is based on the sequence of the RNA
plants. Much less about these viruses is known and for this
polymerase. It is interesting that the various genera do not
reason as well as the fact that no human pathogens are known
group in any obvious fashion related to the host or the vector
among these viruses, they will not be considered further.
for the virus. As one example, note that the orthoreoviruses
are most closely related to the aquareoviruses, not to other
OVERVIEW OF THE FAMILY REOVIRIDAE
viruses of mammals. As a second example, the coltiviruses
are more closely related to the plant reoviruses than to the
other mammalian reoviruses.
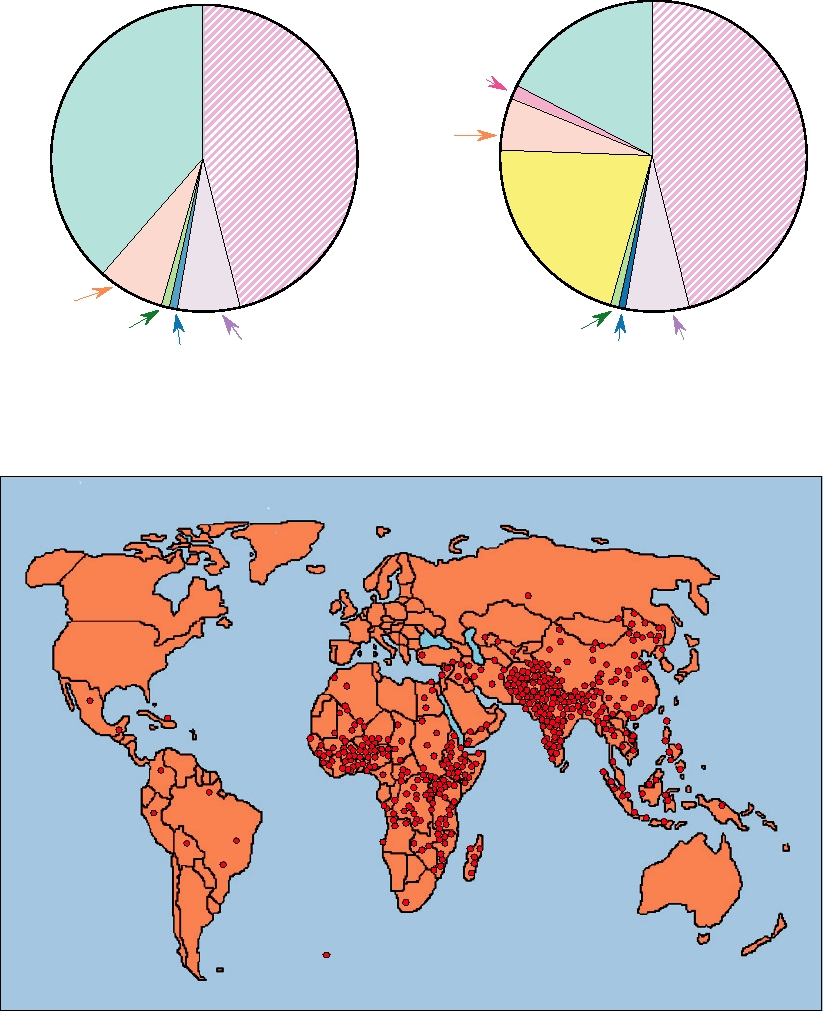
Eleven genera of Reoviridae are listed in Tables 5.1 and
Members of the family Reoviridae replicate in the
5.2 together with a partial listing of viruses in each genus,
cytoplasm. The virion is icosahedral (T=13), 6080 nm in
their hosts and modes of transmission, the diseases they
diameter, and double or triple shelled (the shells consist
cause, and their distributions. A 12th genus, Mycoreovirus,
of protein). The structures of viruses belonging to three
contains viruses that infect fungi. As a taxon, Reoviridae have
genera of the Reoviridae were shown in Chapter 2: reovi-
a wide host range. Members of the genera Orthoreovirus,
rus (genus Orthoreovirus) in Figs. 2.1 and 2.5; rotavirus
Rotavirus, Orbivirus, Coltivirus, and Seadornavirus infect
(genus Rotavirus) in Fig. 2.5; and bluetongue virus (genus
humans as well as other vertebrates, aquareoviruses infect
Orbivirus) in Fig. 2.11 (in this case only the core of the
fish, cypoviruses and entomoreoviruses infect arthropods,
virion is shown). The members of the genus Orthoreovirus
and members of three genera infect plants. Unclassified
have been the best studied and have served as a model sys-
viruses that infect scorpions, crabs, and bed bugs are also
tem for the family, but because of the medical importance
known. Characteristics of the five genera that contain human
of the members of the genus Rotavirus and the veterinary
pathogens are compared in Table 5.3.
TABLE 5.1 Reoviridae of Vertebrates
Virus name
Transmission
World
Genus/members
abbreviation
Usual host(s)
or vector
Disease
distribution
Orthoreoviruses
Non-fusogenic--Subgroup 1
Mammalian reoviruses
MRV
Humans, cattle,
Oralfecal
Gastroenteritis,
Worldwide
types 1, 2, 3
sheep, swine
respiratory disease
Fusogenic--Subgroup 2
Nelson Bay orthovirus
NBV
Flying foxes (bats)
Avian orthoreoviruses
ARV
Birds
Oralfecal
Australia, Worldwide
Wide range of
8 serotypes
symptoms from
inapparent to lethal
Fusogenic--Subgroup 3
Baboon orthoreovirus
BRV
Monkeys
Oralfecal
?
Orbiviruses
Bluetongue
BTV
Sheep, cattle
Culicoides
Rhinitis, stomatitis
Asia, Americas
24 serotypes
Africa, Australia,
African horse sickness
AHSV
Equines
Culicoides
Cardiopulmonary
Africa
10 serotypes
disease
Changuinola
CGLV
Humans
Phlebotamines
Fever
Panama
Kemerovo serogroup:
Kemerovo
?
Humans
E. Europe,
Great Island
GIV
Ticks
Fever, encephalitis
Seabirds
United States
Chenuda
CNUV
Wad Medani
WMV
Domestic animals
Coltiviruses
Colorado tick fever
CTFV
Humans
Ticks
Fever, encephalitis
North America,
Europe
Rotaviruses
Group A
RV-A
Humans, animals
Oralfecal
Infant diarrhea
Worldwide
Group B
RV-B
Humans, animals
Oralfecal
Epidemic adult
Primarily China
diarrhea
Group C
RV-C
Humans, animals
Oralfecal
Clinical significance
Worldwide
unknown
Groups D, E, F, G
Birds, mammals
Oralfecal
Seadornaviruses
Kadipiro
KDV
Vertebrates
Culex and Anopheles
Fever, encephalitis
China, Indonesia
mosquitoes
Banna
BAV
Aquareoviruses
Six serogroups
Fish
?
?
?
Representative members of each genus are shown, and the first listed is the type species of the genus.
TABLE 5.2 Reoviridae of Plants and Insects
Type virus name
Transmission
World
Genus/members
abbreviation
Usual host(s)
or vector
Disease
distribution
Cypoviruses
Cytoplasmic polyhedrosis
BmCPV-1
Arthropods
Ingestion
Diarrhea,
Worldwide
viruses (16 species)
starvation due
to changes in
the gut
Idnoreovirus
Ten-segmented, insect-derived,
DpIRV-1
Arthropods
non-occluded
Fijivirus
Fiji disease virus; five other
FDV
Plants
Delphacid leafhoppers
Australia, Asia,
groups of viruses
South America,
Northern Europe
Phytoreovirus
Wound tumor virus
WTV
Plants
Cicadellid leafhoppers
Rice dwarf virus
RDV
Rice
Cicadellid leafhoppers
Stunting
Southeast Asia, China,
Japan, Korea
Rice gall dwarf virus
RGDV
Oryzavirus
Rice ragged stunt virus
RRSV
Plants (Gramineae)
Planthoppers
Mycoreovirus
3 species of 11 or 12
CpMYRV-1
Fungi
segmented viruses of fungi
Abbreviations: BmCPV-1, Bombyx mori cypovirus 1; DpIRV-1, Diadromus pulchellus idnoreovirus 1; CpMYRV-1, Mycoreovirus 1.
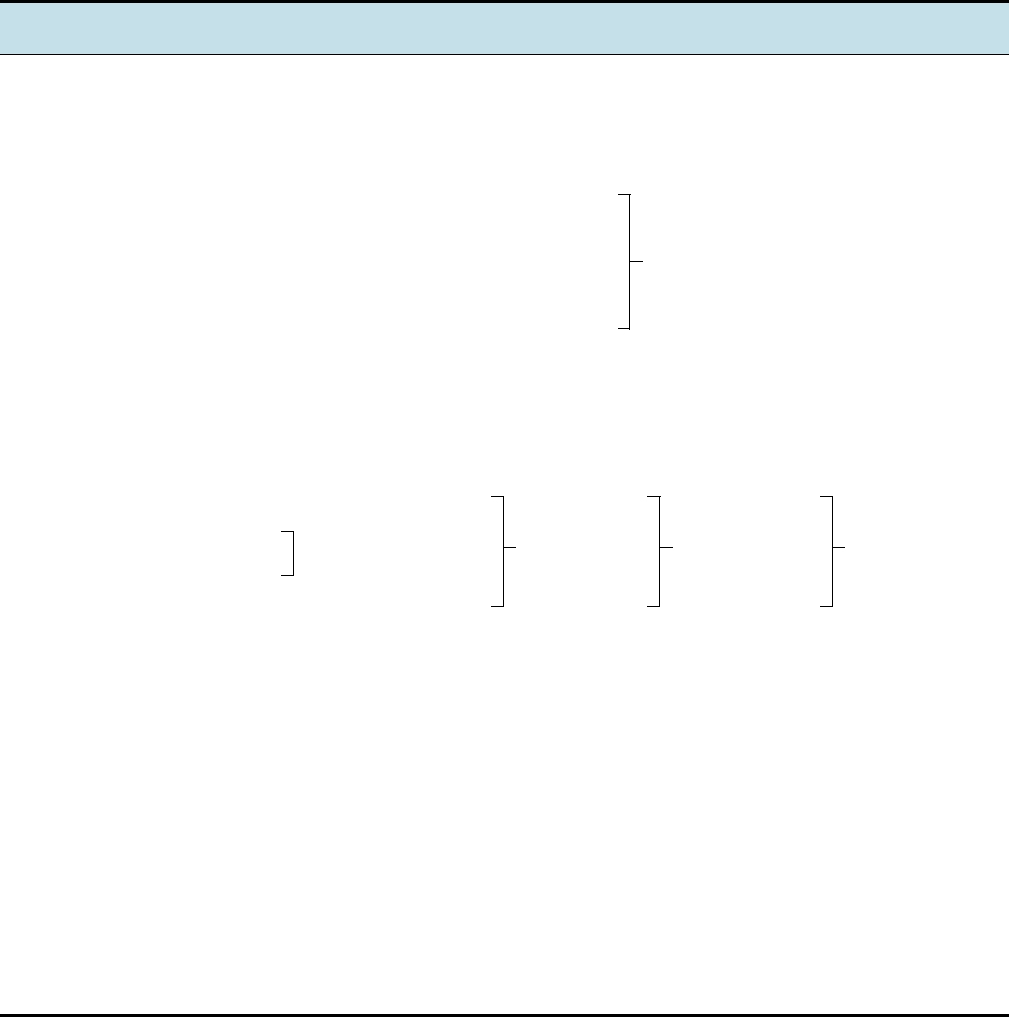
These three orthoreoviruses, now considered strains of a
importance of the members of the genus Orbivirus, these
single species, are virtually ubiquitous viruses of mammals.
viruses have recently come under increased scrutiny.
They have been isolated from many different mammals as
well as from sources such as river water and untreated sew-
Genus Orthoreovirus
age. There is little evidence for host range specificity among
these mammalian viruses. However, reovirus infection of
The genus Orthoreovirus (the "true" reoviruses, to dis-
lower mammals is sometimes associated with more serious
tinguish the genus from the family) contains three viruses
illness than infection of humans.
that infect many mammals, including humans, referred to as
Other branches of the Orthoreovirus genus contain
mammalian orthoreovirus (MRV) types 1, 2, and 3. They
fusogenic reoviruses that infect birds, flying foxes, baboons,
were originally named from the first initials of the words
or reptiles. In general, the avian viruses do not grow in mam-
respiratory enteric orphan virus--they grow in the respi-
malian cells or must be adapted to mammalian cells before
ratory tract and in the enteric tract but were orphans, not
they will grow in them, and thus have a host range distinct
known to cause human illness at the time of their discovery.
from that of the mammalian viruses. The relationships among
The viruses are widespread and the majority of humans have
these viruses are illustrated by the tree in Fig. 5.2. There are
antibodies against all three serotypes by the time they are
five distinct lineages. The three nonfusogenic viruses group
adults. Most infections do not result in symptomatic disease
closely together, consistent with their classification as a sin-
or result in only mild symptoms. Studies with human vol-
gle species, mammalian orthoreovirus (species I). There are
unteers have shown that some individuals develop a mild
several strains of avian orthoreovirus (species II) that form
disease characterized by headache, pharyngitis, sneezing,
a second lineage. Nelson Bay orthoreovirus of flying foxes
rhinorrhea, cough, and malaise.
Comparison of Orthoreovirus, Orbivirus, Rotavirus, Coltivirus, and Seadornavirus
TABLE 5.3
Characteristic
Orthoreovirus
Orbivirus
Rotavirus
Coltivirus
Seadornavirus
Segments
10
10
11
12
12
Size of genome
23.5 kb
19.2 kb
18.6 kb
28.5 kb
20.0 kb
Type virus
Reovirus type 3
Bluetongue-1
Simian rotavirus SA11
Colorado tick fever
Banna
Portal of entry
Oral
Skin
Oral
Skin
Skin
Tissue tropism
Intestinal tract, upper
Hemopoietic
Intestinal tract
Hemopoietic and muscle
CNS
respiratory tract
Vector
None
Culicoid flies, ticks,
None
Ticks
Culex and Anopheles
mosquitoes,
mosquitoes
phlebotomines
Human disease
Upper respiratory
See Table 5.5
Diarrhea, especially in
See Table 5.5
See Table 5.5
infections, infant
children <5 yrs old
enteritis
Consensus terminal nucleotide sequences
5˘GGCUAUUAAAa
5˘(G/C)ACAUUUUGU
5˘GUAU(A/U)(A/U)AA
5˘ Terminal
5˘-GC(U/A)(U/A)
5˘ GU(A/U)AAA
5˘GGC(A/U)NAAAUUb
GAUGUGACC-3˘a
3˘ Terminal
UCAUC-3˘
AC(U/A)UAC-3˘
UGCAGU(G/C)-3˘
(A/G)C(C/U)GAC-3˘
AUAAAAACCC-3˘b
a
Rotavirus A.
b
Rotavirus B.
ROTAVIRUS
Rota "A"
PoRV-A
SiRV
AvRV
BoRV
PoRV-C
Rota "C"
PHYTOREOVIRUS
Rota "B"
RDV-H RDV-A
HuRV
RDV-Ch
KDV
FIJIVIRUS
NLRV
BAV
SEADORNAVIRUS
RRSV
ORYZAVIRUS
CYPOVIRUS
BmCPV
BTV
CTFV
AHSV
COLTIVIRUS
CHUV
GSV
GCRV
MRV-2,3,4 MRV-1
ORBIVIRUS
SBRV CSV
AQUAREOVIRUS
ORTHOREOVIRUS
FIGURE 5.1 Neighbor-joining tree constructed from the amino acid sequences of the RdRp (polymerase) gene of
representatives of 10 different genera of Reoviridae. The size of the oval illustrates the amount of diversion within a
genus, and each oval is color coded for the major host of its members (vertebrates, blue; humans, pink; insects, yellow;
fish, blue-green; and plants, green). Abbreviations are as follows: PoRV, porcine rotavirus; AvRV, avian rotavirus;
SiRV, simian rotavirus; BoVR, bovine rotavirus; HuRV, human rotavirus; NLRV, Nilaparvata lugens reovirus; RRSV,
rice ragged stunt virus; BAV, Banna virus; KDV, Kadipiro virus; BTV, blue tongue virus; AHSV, African horse sickness
virus; CHUV, Palyam virus; MRV-1,2,3,4 mammalian reoviruses types 14; CSV, SBRV, group A aquareoviruses; GSV,
GCRV, group C aquareoviruses; CTFV, Colorado tick fever virus; BmCPV, Bombyx mori cypovirus; RDV-H, RDV-A,
RDV-Ch, isolates of rice dwarf virus. Adapted from Fauquet et al. (2005) Figure 3 on p. 453.
Serotype 1 (Lang)
Mammalian orthoreovirus (I)
Serotype 3 (Deering)
(non-fusogenic)
Serotype 2 (Jones)
Avian orthoreovirus (II)
Fusogenic
NBV Nelson Bay orthoreovirus (III)
reoviruses
BRV Baboon orthoreovirus (IV)
RRV Reptilian orthoreovirus (V)
100 aa changes
FIGURE 5.2 Phylogenetic tree of the orthoreoviruses, derived from amino acid sequences of the σ2 core proteins,
which are encoded in the top three species by the S2 RNA segment, but encoded on a polycistronic S1 segment in the case
of BRV and RRV. The orthoreoviruses are now considered to consist of five species (I to V). A virtually indistinguishable
tree is given by the σNS sequences, and one with only slightly longer arms, but the same topology, by the outer capsid
protein sequences. The scale bar indicates the length of the arm for 100 changes. Adapted from Duncan (1999), and
modified according to Fauquet et al. (2005).
segments of dsRNA (Fig. 5.3), which range in size from 3.9
forms a third lineage (species III). Baboon orthoreovirus
to 1.2 kb and sum to 23.5 kb for MRV-3. The 10 segments
(species IV) and reptilian orthoreovirus (species V) form
fall into three size classes called L for large (3 segments
the remaining two lineages.
called L1, L2, L3), M for medium (3 segments called M1,
The species identified by their alignment in a phyloge-
M2, M3), and S for small (4 segments called S1, S2, S3,
netic tree such as Fig. 5.2 are confirmed by other attributes
S4) (Fig. 5.3 and Table 5.4). Eleven or 12 distinct proteins
that they share, the most important of which is the ability to
are produced, described in more detail later. The proteins
undergo reassortment during mixed infection. Members of
are named by Greek letters corresponding to the L, M, or S
the same species can reassort their genomes during mixed
segment that encodes them, but the numbering of the pro-
infection which, as discussed in Chapter 4 for influenza
teins does not reflect the number of the segment encoding
virus, is important for the evolution of these viruses. A
it (Table 5.4). Of the 11 proteins, 8 are components of the
second feature, which is certainly related to the ability to
virion, 4 in the outer shell and 4 in the inner shell. One
undergo recombination, is conservation of sequences at the
of the viral structural proteins, µ1, is cleaved during virus
ends of the genomic RNAs. For MRV, for example, the 5′
assembly to produce two different products called µ1C and
end of all RNAs begins GCUA and the 3′ end is UCAUC,
µ1N.
whereas the 5′ sequence differs for other species of orthoreo-
viruses. These conserved sequences are probably important
for replication of the RNA and for packaging of the 10 RNA
Entry of Orthoreoviruses into the Cell
segments. These 5′ and 3′ sequences are compared for dif-
After attachment to receptors, normally molecules that
ferent genera of the Reoviridae in Table 5.3.
contain sialic acid, orthoreoviruses are internalized into
Mammalian orthoreovirus serotype 3 (MRV-3) has
endosomes. In endosomes or in lysosomes, proteolysis
been extensively studied as a model for the members of the
of two proteins in the outer shell, σ3 and µ1C, produces
Orthoreovirus genus. In the following discussion, in which
what has been termed an ISVP (infectious subviral particle
aspects of the genome organization, replication, and struc-
or intermediate subviral particle). In this process, µ1C is
ture of orthoreoviruses are described, specific details refer to
cleaved to produce two fragments, δ and a small C-terminal
MRV-3. These details are summarized in Table 5.4.
φ, whereas σ3 is degraded. These cleavages are illustrated
schematically in Fig. 5.4. They can be blocked by agents that
The Genome of Orthoreoviruses
prevent acidification of endosomes, which demonstrates the
importance of the endosomes in the process. ISVPs can also
The orthoreovirus virion is a double-shelled icosahe-
be produced by treating virions with proteases in vitro.
dral particle 85 nm in diameter. The genome consists of 10
TABLE 5.4
Characteristics of the Proteins Encoded by the Ten Genome Segments
of Mammalian Orthoreovirus Serotype 3
Genome
Protein
Segment
Location/molecules of
5˘ NT (nt)
3˘ NT (nt)
segment
product
(length in nt)
ORF (aa)
Function of protein
protein per virion
λ3
L1
3854
18
1267
35
Poly(C) polymerase, catalytic subunit
Core/12
of polymerase/transcriptase
λ2
L2
3916
13
1289
36
Guanylyltransferase
Turrets on core/60
λ1
L3
3896
13
1233
184
NT binding motif, Zn finger
Core/ 120
m2
M1
2304
13
736
83
Putative transcriptase/polymerase
Minor core component/12
component
m1®m1C
M2
2203
29
708
50
Major structural protein
Outer capsid shell/600
mNS(mNSC) 2235
M3
18
719
60
Binds ss RNA
Nonstructural
σ1
S1
1416
12
455
39
HA, neut Ag, cell attachment protein
Capsid/36
σ1NS
120
--
Unknown
Nonstructural
σ2
S2
1331
18
418
59
Structural component
Core/240
σNS
S3
1198
27
366
73
Binds ss RNA
Nonstructural
σ3
S4
1196
32
365
69
Major structural protein
Outer capsid shell/600
Abbreviations: HA, hemagglutinin; neut Ag, contains epitopes recognized by neutralizing antibodies; 5˘ NT, nucleotides at the 5˘ terminus of the RNA
segment that are not translated into protein; 3˘ NT, nucleotides at the 3˘ terminus of the RNA that are not translated.
Source: Joklik and Roner (1996).
Lane
1
2
3
which confirms the importance of proteolytic processing for
the activation of domains required for penetration.
Large (L)
The general scheme of the replication of reoviruses is
Segments
shown in Fig. 5.5. The various steps are discussed in more
detail next.
Medium (M)
Synthesis of mRNAs
Segments
On penetration of ISVPs into the cytoplasm, they are
converted into cores by further loss of δ, φ, and the σ1
fiber, and the rearrangement of the protein λ2 (Fig. 5.4).
Core particles are transcriptionally active: Each of the
10 segments of dsRNA within them is used to synthesize
Small (S)
an mRNA molecule that is the same length as the plus
Segments
strand of the genome segment. These mRNAs are capped
but not polyadenylated. All enzymatic activities required
for the initiation of RNA synthesis, capping, and elon-
gation of the product are present in the core, and occur
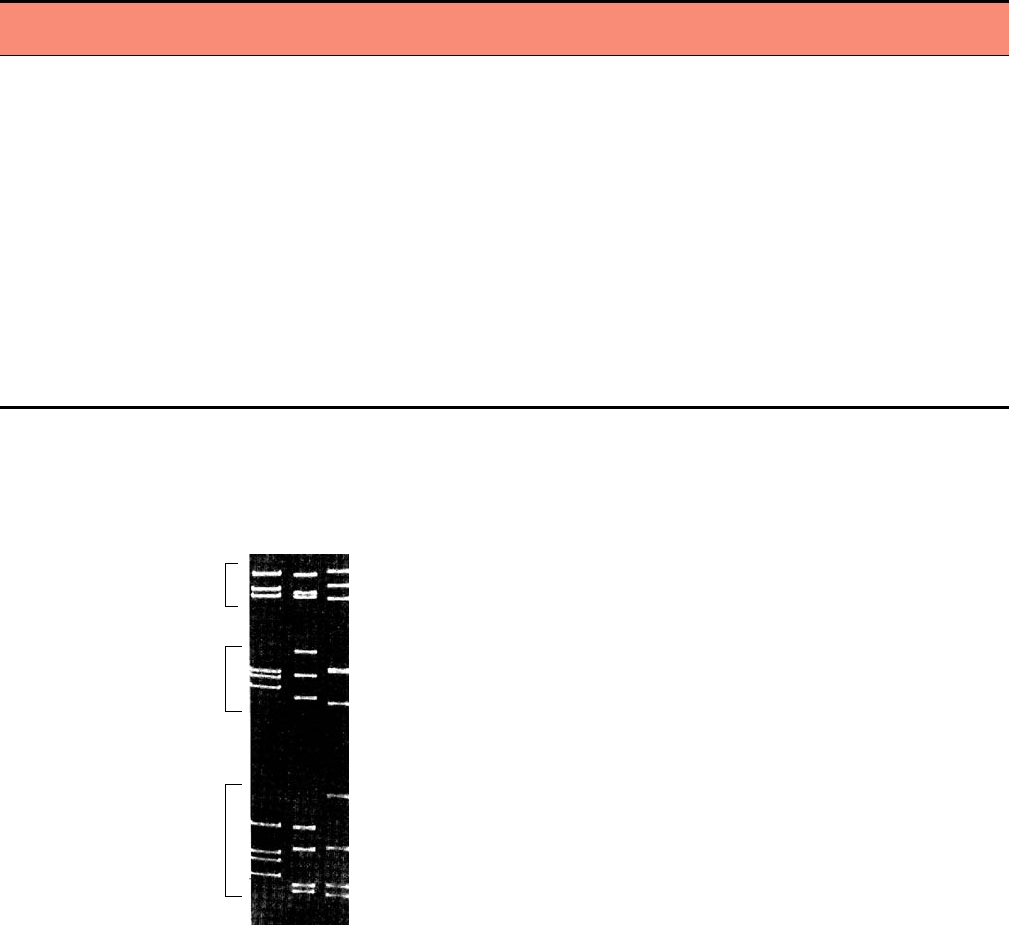
FIGURE 5.3
Gel electrophoresis of the mammalian orthoreovirus RNA
in vitro if cores are supplied with appropriate substrates.
genome segments, showing the variation of the segment size with serotype.
Synthesis of mRNA is conservative: The newly synthe-
Lane 1: reovirus serotype 2 (Jones), Lane 2: reovirus serotype 1 (Lang), and
Lane 3: serotype 3 (Deering). The segments cluster into three groups: 3L
sized mRNAs are extruded from the core and both strands
(large), 3M (medium), and 4S (small). From Fields et al. (1996), p. 1559.
of the parental dsRNA remain within the core. Extrusion
is an active process. Electron microscopic studies have
suggested that the mRNAs are extruded from the 12 ver-
The ISVP is capable of breeching the endosomal mem-
tices of the icosahedral structure. The enzymatic activi-
brane and gaining entry into the cytoplasm. The process of
ties are organized about these 12 fivefold axes, and it has
penetration may involve µ1N, whose N terminus is myris-
been suggested that there is an independent transcription
toylated and lipophilic. When ISVPs are produced by treat-
unit for each genome segment, consistent with the fact
ment of virions with proteases in vitro, they are capable of
that no member of the family Reoviridae has more than
penetrating into the cell by way of the plasma membrane,
12 genome segments.
ISVP
CORE
VIRION
Outer Capsid
Outer Capsid
λ2, µ1C, σ1, σ3
λ2, δ, φ, σ1
λ2
Rearrangement of σ1
Rearrangement of λ2
Cleavage of µ1C to δ and φ
Core
Core
Core
RNA
RNA
RNA
λ3, µ2
Loss of σ3
λ3, µ2
λ3, µ2
Loss of
δ, φ, σ1
λ1, λ3, µ2, σ2
λ1, λ3, µ2, σ2
σ3
Inner Capsid
φ
δ
σ1
Inner Capsid
FIGURE 5.4 Structure of a reovirion and subviral particles derived from it. At left, a cross section through the particle
shows the two protein shells (in yellow and blue) surrounding the RNA (green). In the middle, the intermediate subviral
particle (ISVP) is shown, after the loss of σ3, the cleavage of µ1C to δ and φ, and the extension of σ1. The right illustration
shows the core after the loss of σ1, δ, and φ and the rearrangement of λ2. Redrawn from Niebert and Fields (1995).
outer shell of the virion. Protein σ1 is the cell attachment pro-
Translation of Proteins
tein on the surface of the virion. Trimers of this protein are
In MRV, the 10 mRNAs are translated into 12 proteins
located at the 12 fivefold axes. Interestingly, a complete com-
of which 11 are distinct (Table 5.4). For 8 mRNAs, only
plement of σ1 trimers is not present in all virions. Virions
one reading frame is used and only one protein is produced.
contain from 0 to 12 trimers, with the median number of trim-
For the mRNA produced from segment M3, only one read-
ers being 7. Virions devoid of σ1 are not infectious, but viri-
ing frame is used but two different in-frame AUGs are used
ons containing one or more trimers are infectious.
to initiate translation. Thus, two proteins (µNS and µNSC)
Four proteins of MRV, µNS, µNSC, σ1NS, and σNS,
are produced from this segment that differ only in that the
are nonstructural. µNS, µNSC, and σNS are RNA-binding
longer version has an N-terminal extension. The mRNA
proteins and probably participate in virion assembly. The
from segment S1 is translated using two different, out-of-
function of σ1NS is not known; it is found in the nucleus of
frame AUGs, however, so that two different proteins (σ1
infected cells.
and σ1NS) are produced. In all orthoreoviruses, the mRNA
from the segment corresponding to S1 is translated into two
or three different proteins. In MRV the ORF for σ1NS is
Assembly of Progeny Virions
completely contained within the ORF for σ1, whereas in
species other than MRV the reading frames for the two or
The mRNAs serve as intermediates in the replication of
three proteins overlap only slightly. The various mRNAs are
reoviruses. Following synthesis and release from the core,
mRNAs quickly become associated with proteins µNS, σNS,
translated with widely different efficiencies so that different
and σ3 to form complexes containing single-strand RNA. All
amounts of the 11 or 12 proteins are produced.
complexes contain µNS, but only half contain σ3 and one-
Of the proteins produced, eight are components of the
quarter contain σNS. Complexes containing dsRNA appear
virion and the rest are nonstructural, present only within the
infected cell. Proteins λ1 and σ2, present in 120 and 240
later, which contain the three proteins just named but also
copies, respectively, form the shell of the core. Proteins λ3
contain protein λ2 and the RNA polymerase. Significantly,
and µ2 are present in 12 copies within the core, at the 12
all 10 dsRNA genome segments are present in equimolar
fivefold axes. Protein λ3 is the catalytic subunit of the RNA
quantities in these complexes, suggesting that the selection
polymerase, and µ2 is believed to be a component of this
and assortment of the 10 genome segments into progeny viri-
enzyme complex.
ons is associated with the conversion of (+)RNA into double-
Pentamers of protein λ2, present in 60 copies, form "tur-
stranded RNA. Because the particle-to-infectious virus ratio
rets" at the 12 fivefold axes of the core through which the
is almost one, the assembly process is clearly precise.
During maturation, protein µ1 undergoes a cleavage to
mRNAs are extruded. This protein has guanyltransferase
produce two fragments called µ1N (the N-terminal fragment)
activity and is a component of the complex that caps the
mRNAs. Protein µ1 and its cleavage products, µ1N and µ1C,
and µ1C (the C-terminal fragment). Fragment µ1N is small
together with protein σ3, both present in 600 copies, form the
(4 kDa) and myristoylated, and this process bears a striking
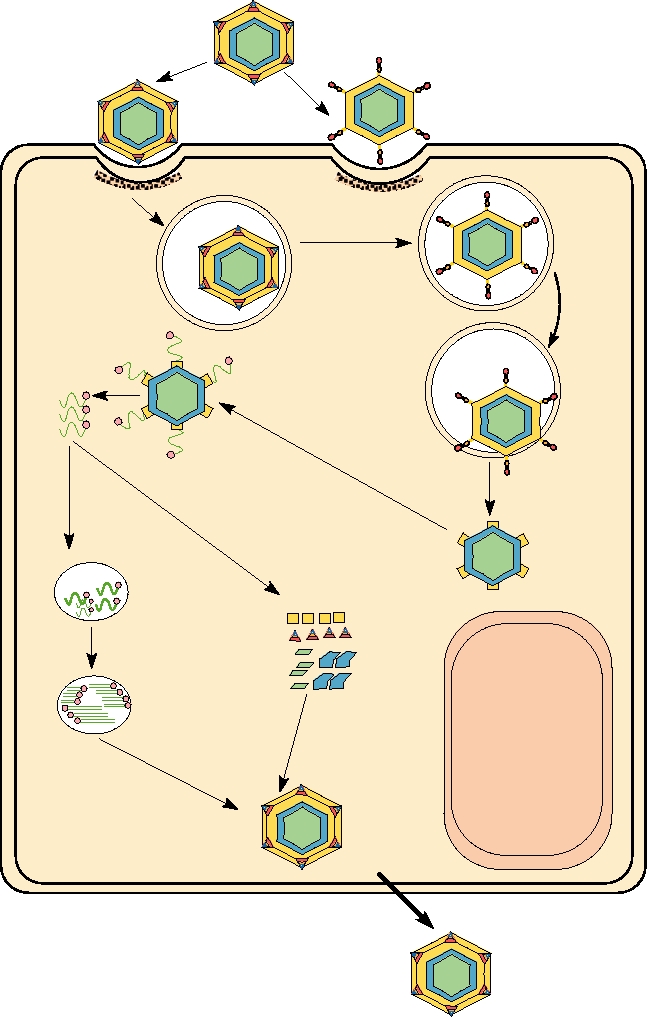
Virion
ISVP
Virion
Proteolysis
Attachment
Endocytosis
Proteolysis
Capped
transcripts
Primary
transcription
Penetration
RNA
Assortment
Translation
Core
RNase-sensitive
Complex
Minus-strand
Synthesis
RNase-resistant
Complex
Capsid
Assembly
NUCLEUS
Progeny
Virion
Release
FIGURE 5.5 The reovirus replication cycle. The primary steps are labeled in blue, while the intermediate products are
labeled in black. This diagram was adapted from Figure 6 in Fields et al. (1996) on page 1706. The color coding of the
protein components is the same as in Fig. 5.4.
similarity to the cleavage event that occurs during the matu-
activities induced by interferon. Thus, the sequestering of the
ration of picornaviruses (see Chapters 2 and 3).
dsRNA within particles where it is not available for interaction
It is significant that the dsRNA is never free but always
with cellular sensors that detect the presence of dsRNA or with
present in particles. As described in Chapter 10, dsRNA is a key
enzymes that are activated by binding dsRNA is important for
molecule in both the induction of interferon and in the antiviral
avoiding this potent cellular defense against viral infection.
Reoviruses and Cancer
rotavirus particle resembles a wheel with spokes from which
it derives its name (rota = wheel in Latin). The assembly
One fascinating recent development is the possibility
of rotaviruses differs in one important detail from that of
that orthoreoviruses might be useful in controlling some
orthoreoviruses. Subviral particles are assembled in the
cancers. They selectively kill mammalian cells whose Ras
cytoplasm and bud through the endoplasmic reticulum,
signaling pathways have been activated. This comes about
acquiring an envelope that is subsequently lost. During this
because orthoreovirus replication is constrained by the inter-
maturation process, the outer capsid layer of the virion is
feron stimulated gene RNA-activated protein kinase (PKR)
acquired. Perhaps because of this transient association with
(Chapter 10). However, tumor cells whose Ras pathways
membranes, two of the rotaviral proteins are N-glycosylated
are activated lack PKR activity, allowing the virus to rep-
and one is O-glycosylated. In contrast, no orthoreovirus
licate vigorously. Since nearly two-thirds of human tumors
protein is known to be glycosylated.
may have an activated Ras pathway, and since orthoreovirus
Activation of infectivity of the virion requires cleavage
infection is fairly benign, it is possible that MRV could be
of one of the major outer capsid proteins, similar to the
used to control or eradicate some tumors.
case for the orthoreoviruses where cleavage of an external
protein also occurs. In rotaviruses this process normally
Genus Rotavirus
occurs in the enteric tract where the virus is exposed to
secreted cellular proteases. Uncleaved rotavirus will bind
Rotaviruses are viruses of higher vertebrates and are
to cells but cannot penetrate and virus produced in cultured
very widely distributed. They cause gastroenteritis in their
cells must be treated with proteases to render it infectious.
various hosts and many different serotypes are known.
The protein is cleaved at a hydrophobic sequence that is
They have been isolated from monkeys, cattle, dogs, cats,
postulated to possess fusion activity, analogous to the proc-
pigs, sheep, horses, chickens, and turkeys, as well as from
ess that occurs in enveloped viruses such as influenza virus
humans. Viruses isolated from different animals exhibit
where precursor glycoproteins must be cleaved to activate
extensive serological cross-reactivity but limited ability to
fusion activities.
replicate in other hosts. As one example, rotaviruses isolated
from monkeys and cows can infect humans but cause much
milder symptoms than human rotaviruses, and have been
The Human Rotaviruses
examined for use as vaccines.
The rotaviruses cause diarrhea, primarily in newborns
Rotaviruses have been assigned to five species to the cur-
and the young, and the human rotaviruses are the single
rent time, rotavirus A, B, C, D, and E. Two tentative spe-
most important cause of severe diarrheal diseases of infants
cies may also be recognized in the future, rotavirus F and
and young children. In one study in the United States, non-
G. Assignment to species is based on sequence identities
bacterial infectious gastroenteritis, of which rotaviruses
and, where known, on the ability of members of a species
account for about half (Fig. 5.6), was found to be the sec-
to undergo reassortment during mixed infection. Human
ond most common disease in humans, accounting for 16%
infection is caused by viruses in rotavirus A, B, and C.
of illnesses over a 10-year period and occurring on average
1.5 times per person per year. Severe diarrhea can result in
Rotavirus Structure and Replication
dehydration that can be fatal if fluids are not replaced. In
Rotaviruses contain 11 genome segments that sum to
the developing world where hospitalization is not readily
18.6 kb (Table 5.3) and which are simply numbered from
available, diarrheal disease, about half of which is caused
1 to 11 in order of decreasing size. As for orthoreoviruses,
by rotaviruses (Fig. 5.6), are a major cause of infant mor-
one segment encodes two proteins in overlapping reading
tality. Firm estimates of the extent of diarrheal disease in
frames and the mRNA transcribed from another segment is
developing countries are difficult to establish, but in Asia,
translated into two proteins using two in-frame start codons
Africa, and Latin America there may be more than 1 bil-
so that they differ only in that one is a truncated form of the
lion cases of diarrhea each year with 23 million deaths.
other. Of the 12 proteins that differ in primary sequence, 6
The majority of deaths occur in children less than 5 years
are structural and 6 are nonstructural. Proteins are numbered
of age, where 14% of diarrheal episodes are fatal. Overall
in order of decreasing size as either virion structural proteins
it has been estimated that rotaviral disease causes between
(VP1-8, where VP4 is cleaved to produce VP5 and VP8) or
300,000 and 800,000 deaths worldwide each year. The glo-
nonstructural proteins (NSP1-6).
bal distribution of deaths caused by rotaviruses is illustrated
The replication of the virus and the overall structure of
in Fig. 5.7.
the virion resemble those of the orthoreoviruses, but with
Replication of rotaviruses is normally confined to termi-
some exceptions. Virions are triple shelled rather than dou-
nally differentiated enterocytes that line the tips of micro-
ble shelled and the virion is distinguishable from that of
villi in the small intestine. How the deaths of these cells
orthoreoviruses in the electron microscope (Fig. 2.5). The
provokes diarrhea has not been definitively established,
Developed Countries
Third World
Parasites
Unknown
1.4%
18%
Rotavirus
Unknown
Other
Rotavirus
5.5%
46%
39%
Bacteria
46%
Toxigenic
E. coli
21%
Bacteria
6.9%
Astrovirus
0.7%
Astrovirus
Adenovirus
Adenovirus
Calicivirus
0.7%
Calicivirus
6.7%
6.7%
0.7%
0.7%
FIGURE 5.6 An estimate of the role of various etiologic agents in severe diarrheal disease requiring hospitalization in
infants and young children in developed countries and in the Third World. Adapted from Kapikian (1993).
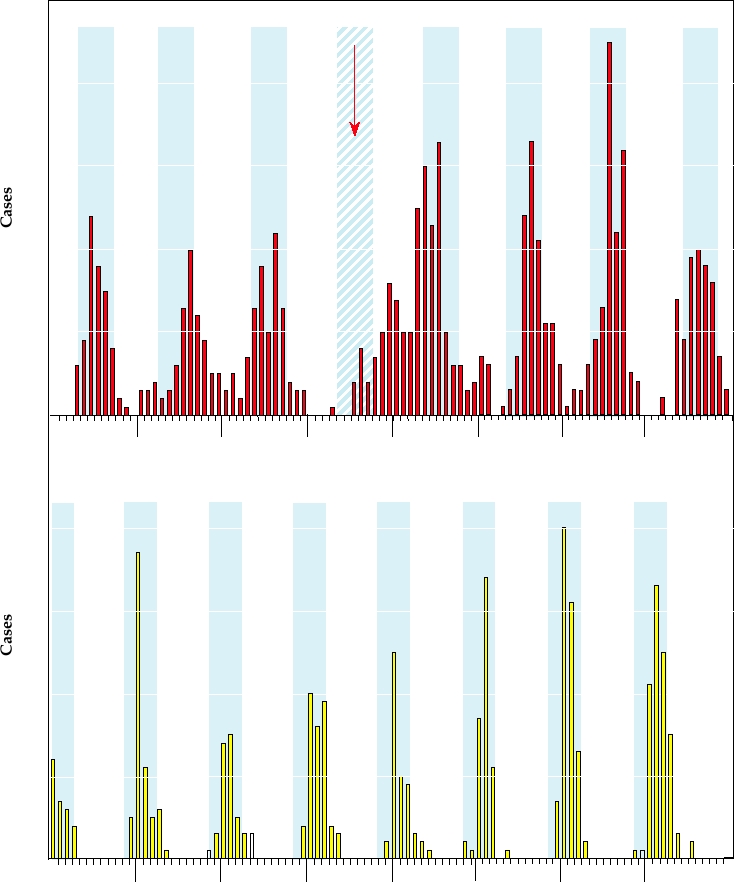
each dot = 1000 deaths
FIGURE 5.7 Deaths (estimated) from rotavirus in 2003 in children under 5 years old. Adapted from Glass (2006).
The 10 countries that suffered the greatest losses are Ethiopia (28,905); Nigeria (47,525); the Democratic Republic of
Congo (28,905); Tanzania (11,440); India (146,044); Bangladesh (18,986); Indonesia (14,064); China (41,076); Pakistan
(36,450); and Afghanistan (17,830). These countries together account for about two-thirds of the worldwide deaths.
although maladsorption and osmotic diarrhea are thought to
confined to replication in the enteric tract, recent studies
be involved. Rotaviral diarrhea is watery, suggesting either
have shown that rotavirus infection may result in systemic
a secretory process or an osmotic process. It is also known
infection, albeit rarely.
that one of the viral proteins, NSP4, is an enterotoxin that
In temperate climates, epidemics of rotaviral disease occur
will induce diarrhea when given to mice. Although normally
in the winter. This is illustrated in Fig. 5.8 for epidemics in
50
Melbourne
Drought
40
30
20
10
0
JSN
JM M
JSN
JM M
J
JM M
J SN
JMM
JSN
J MM
J SN
JMM
J S N JMM J S
JMM
SN
N
1980
1981
1982
1983
1984
1985
1986
1987
Washington, D. C.
40
30
20
10
J MM
JSN
JM M
JSN
JMM
JSN
JMM J S N
JMM J S N
JM M
J
S N JMM
J SN
JMM
JSN
1975
1976
1977
1978
1979
1980
1981
1982
FIGURE 5.8 Monthly rotavirus infections over a multiyear period. Seasonal fluctuations are clear, with the cooler
months shaded in blue. Upper panel shows hospitalizations for gastroenteritis in Melbourne, Australia. The lower panel
shows hospitalizations in a children's hospital in Washington, D.C. Note the anomalous pattern for Melbourne in 1983,
a year in which there was a severe drought. Data from Barnes et al. (1998) and Brandt et al. (1983).
Melbourne, Australia, and in Washington, D.C. In southern
Rotavirus Vaccines
Australia, epidemics peak in the JuneSeptember time frame
Because of the seriousness of rotaviral disease on a world-
(their winter), while epidemics peak in the JanuaryMarch
wide basis, there have been ongoing efforts to develop vac-
time frame in the eastern United States. In the United States,
cines against rotaviruses. Such vaccines have been directed
epidemics peak in November and December in the warmer
at newborns and infants, in whom the problem is most
southwestern states and move to progressively later months
acute. Vaccine development and interpretation of results
as they spread to cooler states north and east (Fig. 5.9). As
from clinical trials have been complicated by several fac-
is the case for seasonality in epidemics of disease caused
tors, including the possible presence of maternal antibodies
by other viruses, the reasons for the association of rotaviral
in newborns, the fact that rotaviral infections often do not
outbreaks with cool weather are poorly understood.
Nov
Dec
Jan
Feb
Mar
Apr
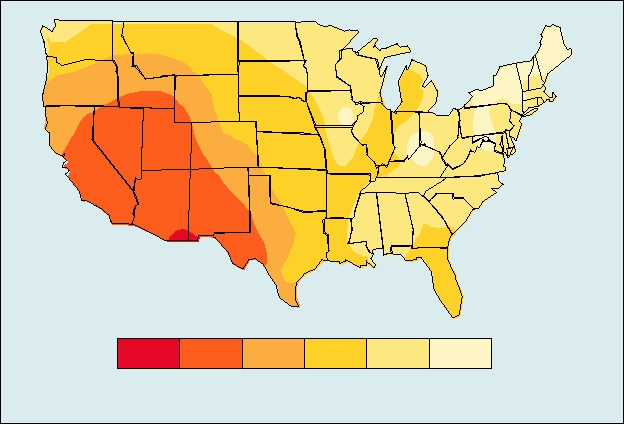
FIGURE 5.9 Average time of peak rotavirus activity in the contiguous 48 states, United States, using cumulative data
from July 1991 to June 1997. This contour plot was derived using the median value for time of peak activity reported by
each regional or state diagnostic laboratory. The surveillance system and analytic methods used to create this map are
described in greater detail in Török et al. (1997).
produce absolute and lasting immunity to subsequent rein-
severe rotaviral disease in newborns and the very young.
fection, and the fact that there are multiple serotypes of rota-
This vaccine (RotaShield) was licensed in 1998 for general
viruses that infect humans. There are four major serotypes
use in the United States. It was anticipated that this vaccine
of rotaviruses that cause widespread and serious infections
would be useful not only in the developing world where
of humans, and thus recent efforts have been directed toward
rotavirus infection causes many deaths in infants, but also in
developing a quadrivalent vaccine that would protect against
the developed world where rotaviral infections lead to many
all four serotypes. Furthermore, the objective of vaccination
cases of diarrhea each year that require hospitilization or vis-
has now been defined as the prevention of severe rotaviral
its to physicians. However, on widespread use in the United
disease in very young children rather than prevention of any
States, it was found that a small number of infants developed
rotaviral disease.
the bowel obstruction called intussusception after immuni-
With these objectives, a rotavirus vaccine was developed
zation. This obstruction sometimes clears spontaneously but
using a virus from another animal species, the rhesus rotavi-
can require a fairly benign treatment by medical personnel.
rus, as a human vaccine. The rhesus rotavirus replicates well
In a small minority of cases, surgery is required to correct
enough in humans to elicit an immunizing response but does
the defect. The vaccine was subsequently withdrawn.
not cause serious illness, and the use of this virus as a human
The withdrawal of the rotavirus vaccine raises interest-
vaccine has been compared to the use of cowpox virus by
ing legal and moral questions, and illustrates the difficul-
Jenner to immunize against smallpox (Chapter 7). The rhe-
ties associated with developing and introducing vaccines.
sus rotavirus will successfully immunize people against only
Roughly 1 out of 2000 infants develop intussusception in
one of the four major rotavirus serotypes, and not against
the first 2 years of life. It seems clear that vaccination with
the others. However, rotaviruses contain 11 genome seg-
the rotaviral vaccine triggers intussusception in a small
ments and the different segments readily undergo reassort-
fraction of vaccinees, since immunization with RotaShield
ment when tissue culture cells are infected by more than
increased the risk of intussecption 20- to 30-fold within the
one strain, that is, genome segments can be interchanged in
first 2 weeks after vaccination, and one in 10,000 vaccinees
progeny viruses. This property was used to isolate reassorted
developed the condition immediately after immunization.
rhesus rotaviruses in which all of the segments were derived
However, it is not known whether vaccinated infants are
from rhesus rotavirus except for the segments encoding the
more likely to develop the obstruction in the first 2 years of
surface proteins, which were derived from human viruses
life than are nonvaccinated infants. It is even possible that
of the other three major serotypes. Clinical trials showed
the vaccine is actually protective in that fewer vaccinated
that a quadrivalent vaccine based on rhesus rotavirus and
infants will ultimately develop intussusception than non-
three reassorted rotaviruses, so that all four serotypes impor-
vaccinated infants. Furthermore, in the United States, about
tant for humans were present, was successful at preventing
55,000 children are hospitalized every year for rotaviral
disease and 20 to 40 die. Use of the vaccine in the United
ticks. Members of five species, including African horse
States would drastically reduce hospitalizations and the
sickness virus, bluetongue virus, and epizootic hemorrhagic
death rate would probably decline since intussusception is
disease virus, cause disease in domestic animals, and mem-
almost never fatal with proper treatment. However, because
bers of four other species cause human disease. Human dis-
of the legal atmosphere in the United States and the ethical
ease caused by naturally acquired orbiviruses may require
dilemma of giving a problematical vaccine for a disease that
hospitalization but is normally not life threatening, and no
is seldom life threatening in developed countries, the vac-
orbivirus is considered to be an important human pathogen.
cine has not been used in the United States since its with-
However, the animal pathogens may cause serious illness in
drawal. Since the vaccine was not used in the United States
domestic or wild animals (Table 5.5).
or other developed countries because of concerns about its
safety, developing nations did not adopt it, despite the fact
Veterinary Pathogens
that the vaccine would undoubtedly have saved hundreds of
thousands of infants from fatal rotaviral infection if it were
African horse sickness virus (AHSV) has caused many
widely used in such countries, where as many as 0.5% of
epidemics of fatal illness in horses in sub-Saharan Africa.
infected children die. A riskbenefit study in Peru, for exam-
The distribution of this virus is illustrated in Fig. 5.10C.
ple, concluded that the vaccine would prevent 1440 deaths
The first recorded epidemic occurred in the Cape Colony
and 23,000 hospitalizations as compared to perhaps 78 cases
in 1719. Thereafter, disastrous epidemics occurred every
of intussusception that might be vaccine related.
20 years or so up until the twentieth century, when vaccines
Now, 8 years and perhaps 5 million deaths later, the
became available. The virus appears to have been endemic,
problem appears to have been solved with the licensing of
presumably in the zebra, but wherever horses were intro-
two new rotavirus vaccines, Rotateq from Merck, licensed
duced, epizootic AHSV was sure to follow. The mortality in
worldwide, and Rotarix from Glaxo Smith Kline, licensed
introduced horses is close to 90%, and AHSV had a major
in Latin America, Africa, Asia, and the European Union. No
impact on agriculture, exploration, and conquest in Africa.
association with intussusception has appeared to date with
The military had to operate without cavalry and the early
the use of these vaccines. These vaccines are also live virus
explorers often walked rather than rode. The virus represents
vaccines, which is important for use in developing coun-
an example of an endemic virus that appears to cause little
tries because they can be given orally. Furthermore, because
disease in its native host (zebra), but which causes very seri-
the viruses replicate in the enteric tract, such vaccines give
ous illness when transmitted to a nonnative host (horses).
rise to IgA that protects the mucosal surfaces infected by
Another important veterinary pathogen is bluetongue
rotaviruses.
virus, which causes a serious disease in sheep. Bluetongue
virus is very widely distributed (Fig. 5.10A) and causes wide-
spread disease, but, interestingly, the strains of bluetongue
Genus Orbivirus
virus in Australia are not pathogenic. Also widespread are
The genus Orbivirus is widely distributed. It contains 21
viruses that cause epizootic hemorrhagic disease in animals
recognized species, each of which contains multiple sero-
(Fig. 5.10B), including outbreaks in deer in North America.
types. As examples, African horse sickness virus contains
9 serotypes and bluetongue virus contains 24 serotypes.
Persistence of Orbiviruses on Erythrocytes
Altogether, more than 150 serotypes of orbiviruses are
currently recognized. Orbiviruses contain 10 segments of
At least some orbiviruses have evolved an unusual way
dsRNA totaling 19.2 kb (Table 5.3) in an icosahedral vir-
of persisting in nature as arboviruses. In tropical areas of the
ion 86 nm in diameter. Reminiscent of rotaviruses, orbivi-
world, arthropods may be continuously active and viruses
ruses may exit the cell by budding through the cell plasma
associated with such arthropods may persist by continuous
membrane to acquire an unstable envelope that is soon lost.
passage between the vertebrate and the invertebrate host.
Viruses can also be extruded from cells without acquiring
However, in many areas of the world, including some tropi-
an envelope.
cal areas, the vector activity may become low or nonexistent
Different orbiviruses infect a wide range of higher ver-
during some periods, such as the dry season or the winter.
tebrates including ruminants, horses and their relatives,
Arboviruses must be able to survive such periods of low vec-
rodents, bats, marsupials, sloths, primates including humans,
tor activity, and different arboviruses have solved this prob-
and birds. Unlike reoviruses and rotaviruses, orbiviruses are
lem in different ways. Most have evolved ways to persist
arboviruses, transmitted by biting flies, mosquitoes, or ticks,
in the invertebrate host during periods of inactivity. Many
and able to replicate in the vector as well as in the vertebrate
can be passed transovarily in the arthropod host, others can
host. Among the vectors known for various orbiviruses are
survive in diapausing insects. Orbiviruses, in contrast, have
insects of the genus Culicoides (midges and gnats), phlebot-
evolved a mechanism to persist in the vertebrate host for long
omine flies, culicine and anopheline mosquitoes, and Ixodes
periods. Viral infection of vertebrates is normally cleared
TABLE 5.5
Representative Orbiviruses, Coltiviruses, and Seadornaviruses Causing Disease
in Humans and Domestic Animals
Genus
Virus
Hosts
Vector
Disease syndromes
Distribution
Orbivirus
African horse sickness
Horse, dog, zebra
Culicoides
Cardiopulmonary disease,
Africa, Asia
(midges)
hemorrhagic fever
Africa, Asia, Australiaa,
Bluetongue
Sheep, cow, goat,
Culicoides
Fever, frothing at mouth,
deer
(midges)
shock, coronitis
Americas
Epizootic hemorrhagic
Deer
Culicoides
Similar to bluetongue
Americas, Australia, Africa
disease
(midges)
Palyam
Cow
Culicoides
Abortion
South Africa, Japan
(midges)
Orungo
Humans
Mosquitoes
Febrile illness
Africa
Changuinola
Humans
Phlebotomines
Febrile illness
Panama
Kemerovo
Humans
Ticks
Febrile illness, encephalitis
Russian, Eastern Europe
Coltivirus
Colorado
Humans
Ticks
Febrile illness, encephalitis,
North America
tick fever
hemorrhagic fever
Eyach
Humans
Ticks
Encephalitis ?
Europe
Seadornavirus
Banna,
Humans
Mosquito
Meningoencephalitis
Southeast Asia, Indonesia, China
Kadipiro
a
Australian isolates of bluetongue virus are not pathogenic.
rapidly by the immune system so that viremia of sufficient
shown in Fig. 5.11. The life cycle of the virus is illustrated in
titer to infect a new arthropod taking a blood meal normally
Fig. 5.12. Although CTF infects many mammals, including
lasts only a few days. However, bluetongue virus becomes
humans, the natural cycle of transmission involves primarily
associated with red blood cells by binding to glycophorins
small mammals and larval and nymphal ticks. Adult ticks may
on the surface of the cell, where it persists in indentations in
transmit the virus to humans and large mammals outside the
the membrane in a nonreplicating state protected from the
normal transmission cycle. Transovarial transmission does not
immune system. The virus evidently can remain attached
occur in ticks and larval ticks are normally infected by feeding
and viable for the life of the erythrocyte. When an arthropod
on small mammals that are viremic. In humans, CTF causes
takes a blood meal, the virus bound to erythrocytes is able
an illness characterized by fever, myalgia, chills, headache,
to initiate infection of the arthropod. Because erythrocytes
and malaise, and 20% of cases require hospitalization. The
have an average lifetime of 160 days, the virus can persist in
acute illness lasts 510 days. Recovery may be uneventful
a viable state in the vertebrate host for many months.
or convalescence may be prolonged for several weeks. CNS
infection or hemorrhagic fever may occur, almost always in
children. Fatalities are rare (<0.1%).
Genus Coltivirus
The second species in the genus is Eyach virus. This
The coltiviruses possess 12 genome segments summing to
virus is widely distributed in Europe where the major host
28.5 kb (Table 5.3). The virion is icosahedral and 80 nm in
is thought to be the European rabbit Oryctolagus cunniculis.
diameter. Like the orbiviruses, the coltiviruses are arboviruses,
It is transmitted by Ixodes ticks and has been associated
transmitted by ticks. Two species are recognized, one found in
with febrile illness and encephalitis in humans. It is hypoth-
North America and the second in Europe. The prototype virus
esized that the virus was introduced into Europe from North
is Colorado tick fever virus (CTF), from which the genus gets
America through migration of lagomorph ancestors from
its name (Colorado tick fever). CTF is present in the Rocky
America to Europe about 30 million years ago.
Mountain area of North America at elevations from 4000 to
10,000 feet, and two serotypes are known. It has been isolated
Persistence of CTF in Erythrocytes
from a number of mammals including humans and from ticks
and mosquitoes that serve as vectors. Transmission to humans
CTF has evolved a way to persist in the vertebrate host
is usually by Dermacentor andersoni ticks whose range is
that resembles that used by bluetongue virus. CTF infects
A.
Bluetongue virus (BTV)
Equator
Pathogenic BTV
Nonpathogenic BTV
B.
Hemorrhagic disease of deer (IHDD), Ibaraki virus, and the
Kemerovo group viruses
Equator
IHDD
Ibaraki
Kemerovo
C.
African Horse Sickness (AHSV)
Equator
AHSV
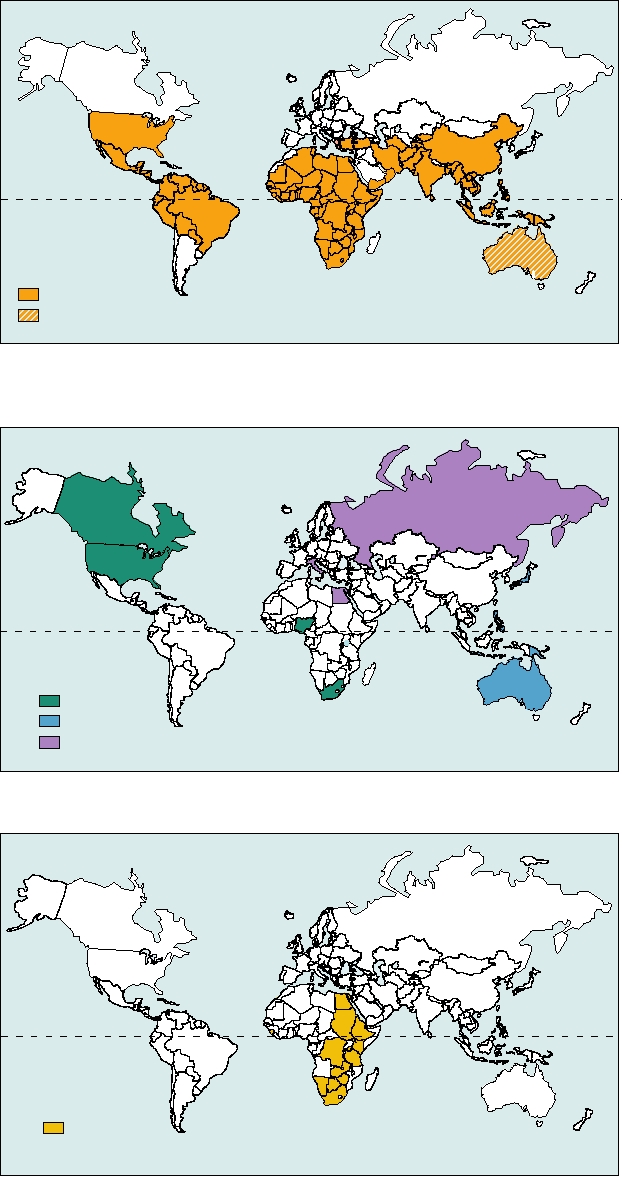
FIGURE 5.10
Geographical distribution of orbiviruses that causes disease in animals and humans. Adapted from
Fields et al. (1996) p. 1736.
British
Columbia
Saskatchewan
Alberta
(1)
Washington
Montana
(130)
Oregon
South
Idaho
(14)
Dakota
Wyoming
(6)
(17)
(88)
Nevada
Utah
Colorado
(255)
(907)
California
(21)
New
Arizona
Mexico
Range of Tick Vector
Dermacentor andersoni
FIGURE 5.11
Distribution of the primary vector of Colorado tick fever, Dermacentor andersoni, shown in color,
and the number of diagnosed human cases of Colorado tick fever in various states between 1980 and 1988. From Tsai
(1991).
bone marrow cells early after infection, including erythro-
recognized, Banna virus, Kadipiro virus, and Liao ning
cyte precursors. The virus remains within the erythrocyte
virus, of which Banna virus was the first isolated and the best
after it matures, in a nonreplicating state, and appears to
characterized. Vectors include Anopheles, Culex, and Aedes
persist for the life of the erythrocyte. When a tick takes a
mosquitoes. Banna has been isolated from humans suffering
blood meal, it can be infected by the virus within the eryth-
from encephalitis or febrile illness, but its association with
rocyte. The differences in the mechanisms used by CTF
human illness has not been conclusively shown since there
and bluetongue virus to persist in the blood of vertebrates
are many viruses in this region that cause such diseases.
are thought to reflect the different vectors used. Culicoides
flies, the vector of bluetongue, take smaller blood meals
Comparison of the Reoviridae with Other
and digest the blood meal in a different way than ticks, the
RNA Viruses
vector of CTF.
Some aspects of the replication cycle of the Reoviridae
resemble those of single-strand (+)RNA viruses (Chapter 3)
Genus Seador navirus
and other aspects resemble those of single-strand (-)RNA
Seadornaviruses contain 12 genome segments and are
viruses (Chapter 4). Replication proceeds through a plus-
transmitted by mosquitoes. The viruses are found in tropi-
strand intermediate that is a messenger RNA and that
cal and subtropical regions of Southeast Asia, primarily
exists, at least transiently, free in the cytoplasm, a charac-
China and Indonesia. The name of the genus is derived from
teristic of (+)RNA viruses. Reoviruses could have origi-
Southeastern Asia dodeca RNA viruses. Three species are
nated from the plus-strand viruses through the acquisition
Search WWH :