RNAs are ambisense in character. The S segment corre-
the lungs, which can result in respiratory death. This loss of
sponds to the bunyavirus S and M segments linked tail to
fluids from the intravascular compartment also leads to an
tail in an ambisense arrangement (Fig. 4.1). The L segment
increase in the hematocrit (the percentage of blood volume
corresponds to the L segments of bunyaviruses but with the
occupied by red blood cells). Early attempts to decrease the
addition of a second gene, encoding a protein called Z, in an
hematocrit by supplying fluid intraveneously simply exac-
ambisense orientation. Expression of the encoded genes fol-
erbated the pulmonary edema. Even with the best treatment
lows an ambisense strategy as described for some of the bun-
today, however, the mortality rate is still very high.
yaviruses. The mRNA for one gene is synthesized from the
It is clear that hantaviruses are widely distributed around
genomic RNA and is expressed early, whereas the mRNA
the world and have been present in their rodent hosts for a very
for the second gene is synthesized from the antigenomic or
long time. Although many are capable of causing serious ill-
vcRNA and is expressed late (Fig. 4.27). As in the bunya-
ness in man, the number of human cases is fortunately small.
viruses, synthesis of arenavirus mRNA occurs in the cyto-
However, there is always the fear that one of these viruses
plasm using a primer that is snatched from cellular mRNAs,
might acquire the ability to spread more readily from human
there is a secondary structure in the RNA between the two
to human and thereby become a more serious problem.
ambisense genes that causes termination of transcription,
and the mRNAs are not polyadenylated.
FAMILY ARENAVIRIDAE
The genomic S RNA is the template for synthesis of the
mRNA for N, and N is therefore expressed early after for the
A listing of the 22 currently recognized arenaviruses is
synthesis of infection. Because N is required for the replica-
found in Table 4.12. They can be grouped on the basis of
tion of the viral RNA, as is the case for all (-)RNA viruses,
sequence alignments and serological cross-reactions into
this arrangement is necessary if the virus is to replicate.
four clades. The Old World viruses form a single clade,
The mRNA for the glycoproteins G1 and G2 is transcribed
whereas the New World viruses group into three different
from the antigenomic copy of S and is therefore expressed
clades, called A, B, C. The genomes consist of two segments
late. The glycoproteins are produced as a polyprotein that is
of (-)RNA which together total about 11 kb. As for other
cleaved in a process that is similar to what happens in the
(-)RNA viruses, the genomic RNA is present in helical
bunyaviruses. There is an N-terminal signal sequence that
nucleocapsids. Budding to acquire the viral envelope is from
leads to the insertion of the precursor called GPC into the
the plasma membrane (Fig. 2.25B). Virions are spherical but
endoplasmic reticulum. The signal sequence is removed by
variable in size, with diameters ranging from 50 to 300 nm.
cellular signalase. The resulting precursor is cleaved by the
It is believed that the number of RNA segments incorpo-
cellular subtilase SKI-1/S1P, the same enzyme that processes
rated into a virus particle is not fixed. Multiple copies of the
the hantavirus glycoprotein precursor, into the N-terminal
genome segments may be present in virions and this may
GN (sometimes called G1 or GP-1) and the C-terminal GC
account, in part if not entirely, for the variation in the size of
(sometimes called G2 or GP-2). GN and GC remain associ-
virions. Also incorporated into the budding virions are vari-
ated as a heterodimer. Only GC has a transmembrane anchor,
able numbers of ribosomes. The name for the family comes
and the process thus resembles what happens in HA of influ-
from the Latin word for sand (arena) because the ribosomes
enza or F of paramyxoviruses where a type I glycoprotein is
in the virions give them a grainy appearance. Why ribo-
cleaved into N-terminal and C-terminal subunits that remain
somes are incorporated into virions is not known, as they
associated by noncovalent bonds.
do not appear to serve a useful function for viral assembly
Producing the glycoproteins late has the effect of delay-
or replication.
ing virus assembly. This allows RNA amplification to pro-
The arenaviruses share many features with the hantavi-
ceed for an extended period of time before it is attenuated by
ruses. They are associated with rodents and have coevolved
the incorporation of nucleocapsids into virions. Attenuation
with them, as have the hantaviruses. They are transmitted to
of RNA synthesis is also effected by the Z protein.
humans by contact with aerosolized rodent urine or feces;
In the case of the L segment, the mRNA for protein L
many cause very serious illness, often hemorrhagic fever,
is produced early by synthesis from the genomic RNA.
with a high mortality rate. Their genome organization and
Proteins L and N are necessary and sufficient for RNA repli-
mode of replication has much in common with the hantavi-
cation, and this orientation of the genes is necessary for virus
ruses, as described later.
replication. The mRNA for protein Z mRNA is transcribed
from the antigenomic and thus Z is expressed late, after rep-
lication of the RNA begins. Z is a small protein of about
Genome Organization and Expression
11 kDa that has multiple functions in viral replication. It has
The genome organization of an arenavirus is illustrated
a RING finger motif and binds zinc. It downregulates RNA
in Fig. 4.27. Arenavirus genomes consist of two segments
replication and the synthesis of mRNAs. It is also required
of RNA, naturally called L(arge) and S(mall). Both genomic
for budding of virions. In fact, expression of Z in the absence
TABLE 4.12 Arenaviridae
Virus name
Natural rodent
Disease in
World
a
host(s)b
Genus/
members
abbreviation
Transmission
humans
distribution
Old World Arenaviruses
Urine, salivac
Aseptic meningitis
Worldwide
Lymphocytic
LCMV
Mus musculus
choriomeningitis
Lassa
LASV
Mastomys sp.
Urine, saliva
Hemorrhagic fever (HF)
West Africa
Mopeia
MOPV
Mastomys natalensis
Urine, saliva
Nonpathogenic?
Mozambique,
Zimbabwe
Mobala
MOBV
Praomys sp.
??
??
Central African
Republic
Ippy
IPPYV
Arvicanthis sp.
??
Nonpathogenic?
Central African
Republic
New World Arenaviruses
Group Ad
Tamiami
TAMV
Sigmodon hispidus
Urine, saliva
Nonpathogenic?
Florida (U.S.)
Whitewater Arroyo
WWAV
Neotoma albigula
Urine, saliva
Three fatal cases of
Western United States
ARDS in Californiae
Bear Canyon
BCNV
Peromyscus californicus
Urine, saliva
??
Western United States
Paraná
PARV
Oryzomys buccinatus
Urine, saliva
Nonpathogenic?
Paraguay
Flexal
FLEV
Oryzomys ssp.
Urine, saliva
Nonpathogenic?
Brazil
Pichinde
PICV
Oryzomys albigularis
Urine, saliva
Nonpathogenic?
Colombia
Pirital
PIRV
Sigmodon alstoni
Urine, saliva
Nonpathogenic?
Venezuela
Allpahuayo
ALLV
Oecomys bicolor and
Urine, saliva
Nonpathogenic?
Northeastern Peru
O. paricola
Group B
Guanarito
GTOV
Zygodontomys brevicauda
Urine, saliva
Venezuelan HF
Venezuela
Junin
JUNV
Calomys musculinus
Urine, saliva
Argentine HF
Argentina
Machupo
MACV
Calomys callosus
Urine, saliva
Bolivian HF
Bolivia
Sabiá
SABV
Unknown
???
Isolated from a fatal case, Brazil
and has caused two severe
laboratory infections
Amapari
AMAV
Oryzomys capito,
Urine, saliva
Nonpathogenic
Brazil
Neacomys guianae
Artibeus spp. bats f
Tacaribe
TCRV
Has been isolated
Nonpathogenic
Trinidad
from mosquito
Cupixi
TCRV
Oryzomys capito
Nonpathogenic
Brazil
Group C
Latino
LATV
Calomys callosus
??
??
Bolivia
Oliveros
OLVV
Bolomys obscurus
??
??
Argentina
a
LCMV is the type virus of the family.
b
Most of these viruses cause chronic infections in their natural rodent hosts.
c
At least one case is known where several recipients contracted LCMV after organ transplants from an asymptomatic donor.
d
White Water Arroyo, Tamiami, and Bear Canyon viruses have nucleoprotein genes related to those of Pichinde and Pirital in Group A, but glycoprotein
genes related to Tacaribe, Junin, and Sabiá in Group B.
e
ARDS, acute respiratory distress syndrome. Until these cases in 19992000, WWAV was not known to cause human illness.
f
Originally isolated from fruit-eating bats, but subsequent isolation attempts from bats have failed.
Source: Fields et al. (1996), Table 1 on p. 1522; Porterfield (1995), Table 11.1 on p. 228; and recent information from Fauquet et al. (2005).
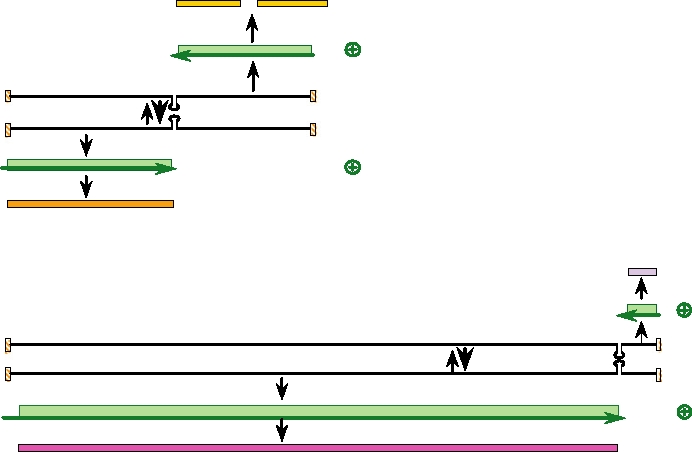
Lassa fever virus S RNA (3417 nt)
Gc (234aa) Gn (256aa)
Translation
Cleavage
39
59
GPC mRNA
mRNA synthesis
39
59
vcRNA
Replication
59
39
Genome RNA
mRNA synthesis
59
NmRNA
3'
Translation
N protein (570 aa)
Z (99aa)
59
Translation
Lassa fever virus L RNA (7279 nt)
Z mRNA
mRNA synthesis
59
39
vcRNA
Replication
59 Genome RNA
39
mRNA synthesis
59
LmRNA
Translation
L protein (2218 aa)
FIGURE 4.27 Genome organization and replication strategy of an arenavirus, Lassa fever virus. The genome consists
of two segments of RNA, L and S. Both segments are expressed using an ambisense strategy. The nucleocapsid protein
mRNA is synthesized from the 3¢ end of the genomic S RNA, while the GPC mRNA is synthesized from the vc S RNA.
A similar strategy occurs with the L segment. In this case, however, more than 95% of the coding capacity is used for the
L protein, the RNA dependent RNA polymerase. Also note that the 5¢ nontranslated region of L mRNA is 157 nt, which
is unusually long for an arenavirus. The Z protein is a so-called "ring finger protein" that is involved in regulation of
transcription and replication. Drawn from data in Lukashevich et al. (1997) and Clegg et al. (1990).
of other viral proteins results in the formation of virus-like
organization and expression, the association with a sin-
particles, and Z has a role in budding analogous to the role
gle rodent species, and the nature of the disease caused in
played by the M proteins of other (-)RNA viruses or the Gag
humans all suggest that the arenaviruses are closely related
protein of retroviruses. It has been found that Z recruits a
to the hantaviruses. A reasonable hypothesis is that the are-
cellular protein called Tsg101 to the site of budding. Tsg101
naviruses arose from the hantaviruses by fusion of the S
has been shown to be required for budding of (at least) two
and M segments to form one segment, which allowed finer
arenaviruses, of HIV, and of Ebola virus. Tsg is a compo-
control of the virus life cycle.
nent of the vacuolar protein sorting machinery of the cell and
The cellular receptor for entry of many arenaviruses is
α-dystroglycan, which plays a critical role in cell-mediated
is therefore active in promoting cellular budding pathways.
Z was originally thought to be a nonstructural protein and
assembly of basement membranes. This protein is widely
was called NS. It is now known to be present in the virion.
distributed in animals and many arenaviruses have a broad
The stoichiometry of proteins in virions of Lassa virus was
tissue tropism. For example, Lassa infection of humans
found to be 1:160:60:60:20 for L:N:GN:GC:Z.
results in high virus titers in spleen, lung, liver, kidney,
heart, placenta, and mammary gland. Viruses that have
a high binding affinity for α-dystroglycan replicate pref-
Natural History and Diseases
erentially in the white pulp of the spleen and infect large
The natural history of the arenaviruses is very similar to
numbers of lymphocytes that are important in the immune
that of the hantaviruses (Table 4.12). They establish a per-
response to viral infection. The ability of these lymphocytes
sistent infection in a single rodent host. Many cause hemor-
to act as antigen-presenting cells results in impairment of
rhagic fever in humans following infection by aerosolized
immune responses resulting in a generalized immunosup-
virus excreted in urine or feces. They appear to have co-
pression. Such viruses are more virulent than those that bind
less avidly to α-dystroglycan. Immunosuppression may be
evolved with their hosts: An evolutionary tree of arenavi-
ruses resembles the tree that describes their rodent hosts, as
important for the establishment of persistent infections in
was true of the hantaviruses. The many similarities in genome
the rodent host, in which the virus does not cause disease.
In humans, however, immunosuppression may lead to much
damage and may be deaf because of such damage. The full
more serious illness.
extent of Lassa disease is not known because most Africans
The arenaviruses can be divided into Old World viruses
infected by the virus do not seek help and there is little
and New World viruses (Table 4.12). Because of their asso-
monitoring of the disease. However, estimates range from
ciation with a single rodent species, their geographic range
100,000 to 300,000 cases per year.
is restricted to that of their host, and rodents have a restricted
Lassa virus was first isolated in 1969 when a nurse in a
range. The exceptions are rodents that have been distributed
rural mission hospital in Nigeria became infected. She was
widely by humans such as the house mouse and the urban
transported to Jos, Nigeria where several health care workers
rat. Many arenaviruses cause hemorrhagic fever in man with
became infected. Serum samples were sent to the United States
significant mortality rates (Table 4.12).
and a well-known virologist at the Yale Arbovirus Research
Unit, Dr. Jordi Casals, became infected with the virus while
working with it and became very seriously ill. He eventu-
Lymphocytic Choriomeningitis Virus
ally recovered but later that same year a technician in another
Lymphocytic choriomeningitis virus (LCMV), the proto-
laboratory at Yale became infected with Lassa fever virus
type virus of the family, is associated with the house mouse
and died, whereupon Yale ceased to work with the virus.
Mus domesticus and Mus musculus. This virus is widespread
The containment facilities in 1969 were not of the quality
in Europe, along with its host, and spread to the Americas
of those in current use and virologists in those days literally
with the (inadvertent) introduction of the house mouse by
took their lives in their hands when working with dangerous
European travelers. LCMV has been intensively studied in
agents. The study of virology owes a great deal to the courage
the laboratory as a model for the arenaviruses, in part because
exhibited by these earlier workers.
it is less virulent for humans than many arenaviruses, and
Lassa has been imported to the United States on at least
in part because its natural host is widely used as a labora-
one occasion in the form of a viremic individual. A resident
tory model for animal work. Mice are small, reproduce rap-
of Chicago attended the funerals of relatives in Nigeria who
idly, and there is a great deal of experience in maintaining
had died of Lassa fever and became infected there. On return
this animal in the laboratory. LCMV is widespread, often
to Chicago he began suffering symptoms of Lassa fever but
being present in colonies of laboratory mice even without
the local hospitals were unable to diagnose the cause of his
overt introduction. It is also present in wild mice and may be
disease, being unfamiliar with it. He eventually died of Lassa
present in pets such as hamsters.
fever, but fortunately there were no secondary cases.
LCMV infection of humans usually results in mild or even
inapparent illness, although serious illness can result with
New World Group B Viruses
occasional mortality. In a recent incident, a woman had been
infected with LCMV from a pet hamster. She suffered no
Several South American arenaviruses belonging to
apparent illness from the viral infection but died of an unre-
Group B are very important disease agents because they
lated cause, a stroke. Her liver, lungs, and kidneys were har-
cause large outbreaks of hemorrhagic fever with high mor-
vested for transplantation. Transplantation of liver, lungs, and
tality rates. The names of a number of these viruses and the
kidney requires immunosuppression so that the transplanted
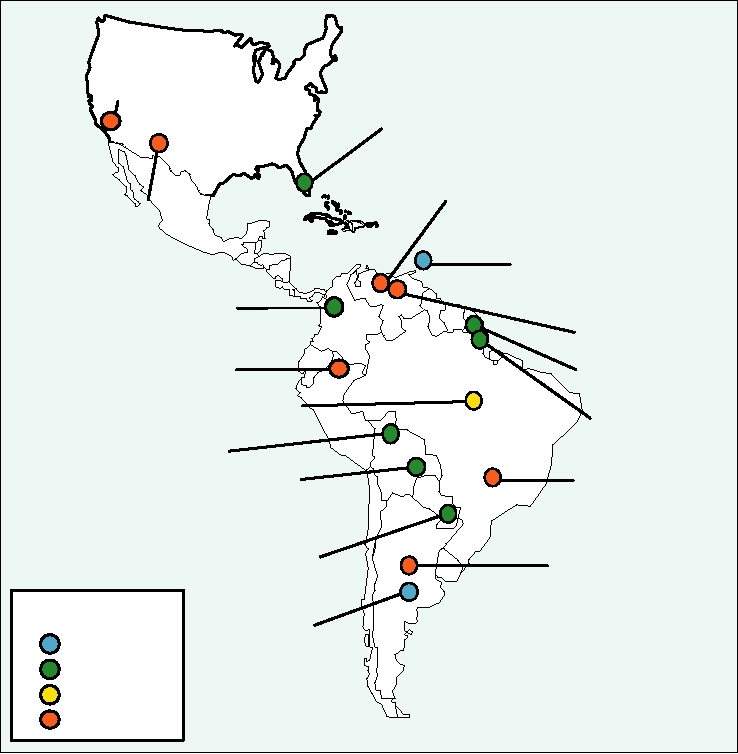
places where they are found are shown in Fig. 4.28. They
organs are not rejected. Three patients receiving the liver,
include Junín virus (causative agent of Argentine hemor-
lungs, and a kidney developed overwhelming infection by
rhagic fever), Machupo virus (Bolivian hemorrhagic fever),
LCMV and died. A fourth patient who received a kidney also
Guanarito virus (Venezuelan hemorrhagic fever), and Sabiá
became quite ill from LCMV infection but survived, aided by
virus (cause of an unnamed disease in Brazil). The diseases
reduction in the immunosuppressive drugs being given.
caused by these viruses are often referred to as emerging
diseases because the number of human cases has increased
with development and expanding populations. The increas-
Lassa Virus
ing number of cases results from development of the pam-
The rodent reservoir of Lassa virus is Mastomys natalensis.
pas or other areas for farming, bringing humans in closer
Lassa virus causes outbreaks in West Africa of an often fatal
association with the rodent reservoirs. Furthermore, the stor-
illness in humans called Lassa fever. The mortality rate aver-
age of grain near human habitation results in an increase in
ages 1015% but may be as high as 60% in some outbreaks.
the local rodent population, and plowing of the fields leads
The virus has a broad tissue tropism and symptoms include
to the production of aerosols which may transmit the dis-
fever, myalgia, and severe prostration, often accompanied
ease to humans. An attenuated virus vaccine against Junín
by hemorrhagic or neurological symptoms. Development of
virus has been developed and is widely used in populations
hemorrhagic symptoms indicates a poor prognosis and death
at risk. The vaccine is effective and has reduced dramati-
often follows. Fatal infection is also characterized by higher
cally the number of cases of Argentine hemorrhagic fever.
viral loads. Survivors of severe infection often suffer nerve
No vaccines are in use for the other viruses, however.
Bear Canyon (2002)
(Peromyscus californicus)
Tamiami (1964)
(Sigmodon hispidus)
Guanarito (1990)
(Zygodontomys brevicauda)
Whitewater Arroyo (1995)
(Neotoma albigula)
Tacaribe (1956)
(Artibeus Bats)
Pichindé (1965)
Pirital (1995)
(Oryzomys albigularis)
(Sigmodon alstoni)
Allpahuayo (1997)
Amaparí (1964)
(Oecomys bicolor)
(Necomys guianae)
Flexal (1975)
(Oryzomys spp.)
Cupixi (1970)
(Oryzomys capito)
Machupo (1963)
(Calomys callosus)
Sabiá (1990)
Latino (1965)
host unknown
(Calomys callosus)
Paraná (1965)
Oliveros (1990)
(Oryzomys buccinatus)
(Bolomys obscurus)
Virus Isolates
Junín (1958)
Before 1960
(Calomys musculinus)
1960 to 1970
1975
1990 on
FIGURE 4.28 Arenavirus isolates in the New World. Also shown are the year of first isolation, and the rodent host of
each virus where known. Adapted from Peters (1998b), Figure 1.
Agents Causing Hemorrhagic Fevers
New World Group A Viruses
in Humans
Three arenaviruses have been isolated in the United
States, Whitewater Arroyo virus, present in the Southwest,
Many viruses, belonging to several different families,
Bear Canyon virus in California, and Tamiami virus,
have been described that cause hemorrhagic fever in humans.
present in Florida (Fig. 4.28). None of these viruses, all
Table 4.13 contains a listing of many of these viruses. These
of which belong to Group A, had been known to cause ill-
viruses include members of the Arenaviridae, Bunyaviridae,
ness in humans until very recently. In 19992000, three
Filoviridae, and Flaviviridae. Many cause severe disease
Californians died following infection by Whitewater
with high mortality, but although the disease is severe, with
Arroyo virus. The disease these three suffered was ARDS
the exception of some arenaviruses, survivors have few
(acute respiratory disease syndrome), although two also
sequelae. The dramatic symptom of profuse bleeding has
had hemorrhagic manifestations. Thus, like the hantavi-
excited the purple prose of many lay authors, best illustrated
ruses, the U.S. arenaviruses may cause isolated cases of
by recent discussions of Ebola virus, and struck terror in
serious illness. There are also a number of Group B viruses
native populations. With the exceptions of yellow fever virus
in South America (Table 4.12), but these are not known to
and Junín virus, there are no vaccines, and treatments are
cause disease in humans.
primarily supportive, although ribavirin therapy holds some
Search WWH :