In this event, the sequences at the two ends of the genome
(VSNJV), and Alagoas. VSIV is the prototype virus of the
or antigenome that encompass these promoters would be
genus and has a genome size of 11,161 nt. The genome is
complementary.
neither capped nor polyadenylated, consistent with the fact
that it is minus-strand RNA.
Host Range of the (-)RNA Viruses
Synthesis of mRNAs
All seven families contain members that infect higher
The VSV nucleocapsid has about 1250 copies of N pro-
vertebrates, including humans. For five of the families, only
tein as its major structural component, leading to the con-
vertebrate hosts are known. The rhabdoviruses and bunyavi-
clusion that each N protein interacts with 9 nucleotides of
ruses, however, have a broader host range. Some are arbo-
RNA. The nucleocapsid also contains about 470 molecules
viruses that replicate in an arthropod vector as well as in a
of P and 50 copies of L. It can synthesize RNA, and P, L,
vertebrate host, and others infect only insects. In addition,
and N are all required for this activity. The organization
some genera of rhabdoviruses and bunyaviruses consist of
of the genome and the production of five mRNAs from
plant viruses. Some of these are transmitted to the plants by
it are illustrated in Fig. 4.2. There is a single polymerase
insect vectors in which the viruses also replicate.
entry site at the 3′ end of the genome, and production of
mRNAs is obligatorily sequential. Synthesis begins at the
exact 3′ end of the genome and a leader RNA of 48 nucleotides
FAMILY RHABDOVIRIDAE
is first synthesized. The leader is released and synthesis of the
The genome organization of the rhabdoviruses is the
first mRNA, that for N, is initiated. The RNA polymerase com-
simplest of the (-)RNA viruses and it is useful to begin our
plex has capping activity, and the mRNA is capped during or
coverage with this group. The genome is a single piece of
shortly after initiation. At the end of the gene for N, the tran-
minus-strand RNA 1115 kb in size. The genomes of all
scriptase reaches a conserved sequence AUACUUUUUUU,
rhabdoviruses contain five core genes, called N, P, M, G, and
where it begins to stutter and produces a poly(A) tract at the
L in that order in the genome reading 3′ to 5′, which result
3′ end of the mRNA. The polymerase complex will not termi-
in the production of five to seven proteins, five of which are
nate or stutter unless the conserved AUAC is present imme-
present in the virion. Some rhabdoviruses contain only these
diately upstream of the U7 tract, and the sequence AUACU7
five genes, but others contain one to five extra genes inserted
is therefore a consensus termination-polyadenylation signal.
in various regions of the genome. The animal rhabdoviruses
The capped and polyadenylated mRNA for N is terminated
are bullet shaped, approximately 200 nm long and 75 nm in
and released, the transcriptase skips the next two nucleotides,
diameter (Fig. 2.23), whereas some of the plant viruses are
which are referred to as the intergenic sequence, and initi-
bacilliform, being rounded at both ends. The rhabdoviruses
ates synthesis of the second mRNA, that for P, at the con-
infect mammals, birds, fish, insects, and plants, and are pres-
served gene start signal UUGUC. Following synthesis of this
ently divided into six genera. A listing of these genera and a
mRNA, the polymerase again stutters at the oligo(U) tract in
representative sample of the viruses in each genus, together
the AUACU7 signal to produce a poly(A) tract, releases the
with several characteristics of each virus, are shown in Table
capped and polyadenylated mRNA, skips the next two nucle-
4.2. Members of three genera infect mammals, namely, the
otides, and begins synthesis of the third gene, that for M. The
vesiculoviruses (type virus: vesicular stomatitis Indiana
process continues in this way through the fourth gene (the G
virus), lyssaviruses (type virus: rabies virus), and ephemero-
protein) and the fifth gene (the L protein, L for large because it
viruses (type virus: bovine ephemeral fever virus). The
comprises about 60% of the genome). In this way, five capped
novirhabdoviruses infect fish, and the cytorhabdoviruses and
and polyadenylated mRNAs are produced. In VSV, the inter-
nucleorhabdoviruses infect plants. Some or all of the mem-
genic sequence is always two nucleotides. After releasing the
bers of four genera are transmitted by arthropods (Table 4.2).
L mRNA, the polymerase complex terminates synthesis some
50 nucleotides before the 5′ end of the genome is reached.
In addition, a large number of the more than 175 currently
known rhabdoviruses have not been assigned to a genus. The
As described earlier, synthesis of the mRNAs proceeds in
animal rhabdoviruses replicate in the cytoplasm, but certain
strict sequential order and the attenuation that occurs at each
of the plant rhabdoviruses may replicate in the nucleus.
initiation step results in a gradient in the amounts of mRNAs
produced. This attenuation appears to be important for regu-
lation of the virus life cycle, so that the mRNAs for proteins
Genus Vesiculovirus
needed in most abundance are produced in most abundance.
Vesicular stomatitis virus (VSV) has been extensively
Reorganization of the genome to change the order of genes
studied and serves as a model for the replication of (-)RNA
gives rise to viable virus, but the yield of such virus during
viruses in general and rhabdoviruses in particular. Three
an infection cycle in cultured cells, and thus the fitness of the
serotypes have been recognized, Indiana (VSIV), New Jersey
virus, is reduced.
TABLE 4.2 Rhabdoviridae
Virus name
Transmission/
World
a
Genus/members
abbreviation
Usual host(s)
vector?
Disease
distribution
Vesiculovirus
Vesicular stomatitis
VSIV
Humans, horses,
Airborne, Insects?
Vesicles on
Americas
Indiana
ruminants, swine
tongue and lips
Chandipura Virus
CHPV
Mammals, including
Sandflies
Febrile illness
India, Asia?
humans
Piry
PIRYV
Mice, humans
Sandflies
Febrile illness
Brazil
Lyssavirus
Rabies
RABV
Humans, dogs, skunks,
Infectious saliva
Malaise, then
Worldwide except
foxes, raccoons
delirium, then
some islands, and
coma and death
Australia
ABLV,b EBLV,
Bats, humans
Infectious saliva
Like rabies
Europe, Africa,
Bat lyssaviruses
LBV
Australia
Mokola
?
Humans, dogs, cats,
?
Like rabies
Africa
shrews
Ephemerovirus
Bovine ephemeral fever
BEFV
Cattle, water buffalo
Hematophagous
Fever, anorexia
Africa, Asia,
arthropods
Australia
Adelaide River
ARV
Cattle
Berrimah
BRMV
Cattle
Novirhabdovirus
Infectious hematopoietic
IHNV
Salmonid fish
Waterborne,
Hemorrhage
Pacific Northwest of
necrosis (and other
contaminated
North America
fish viruses)
eggs
Cytorhabdovirus
Lettuce necrotic yellows
Plants
Aphids
?
?
?
Northern cereal mosaic
Plants
Leafhoppers
Strawberry crinkle
Plants
Aphids
Nucleorhabdovirus
Potato yellow dwarf
Plants
Leafhoppers
Maize mosaic
Plants
Leafhoppers
Sonchus yellow net
Plants
Aphids
a
Representative members of each genus are shown, and the first virus listed is the type species.
b
Virus name abbreviations: ABLV, Australian bat lyssavirus; EBLV, European bat lyssaviruses -1 and -2; LBV, Lagos bat virus.
The mRNAs for N, M, G, and L are each translated into
Replication of the Genome
a single protein. That for P is translated into three proteins.
Synthesis of viral proteins, in particular of the N protein,
The major translation product of this mRNA is P, which is
produced using an initiation codon near the 5′ end of the
allows the enzymatic activity present in the genomic nucleo-
capsid to switch from synthesis of messengers to replication
mRNA. Initiation of translation also occurs at two down-
of the genome. Replication requires producing a full-length
stream AUGs. These two downstream AUGs are in frame
antigenomic template, and the immediate encapsidation of
with one another but in a different reading frame from P. Use
the newly synthesized (+)RNA into plus-strand RNP contain-
of these alternative AUGs leads to the synthesis of short pro-
ing N, P, and L during synthesis is required. In the absence
teins of 55 and 65 amino acids (of which the shorter protein
of N for encapsidation, the system defaults to synthesis of
is a truncated version of the longer one). The functions of
mRNAs. The M protein also appears to regulate RNA synthe-
these small proteins have not been established for VSV, but
sis. In the replication mode, the polymerase complex ignores
extra products translated from the P gene of the paramyxovi-
all of the initiation, termination, and polyadenylation signals
ruses are known to interfere with host defense mechanisms,
utilized to produce mRNAs, and instead produces a perfect
as described later.
A
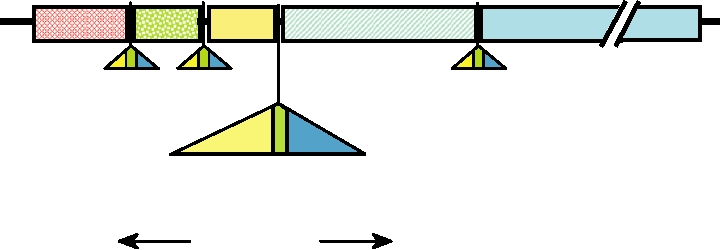
Location of intergenic sequences of VSV (a rhabdovirus), and detailed view of the M/G intergenic region
M
L
N
P
G
le
tr
EI
S
5
3
5
...AUAGGGAUACUUUUUUUGAUUGUCUCUAG...
Genome RNA
m
Gp
AACAGAGAUC...3
pp
N
...UAUCCCUAUG poly(A)
mRNAS
M mRNA
G mRNA
B
Genomic sequences at other intergenic regions in the VSV genome
5
3
...CGAUGUAUACUUUUUUUGAUUGUCUAUAG...
/P
...CAUCUGAUACUUUUUUUCAUUGUCUAUAG...
P/M
...UUAAAAAUACUUUUUUUGAUUGUCGUUAG...
G/L
FIGURE 4.2 (A) Schematic diagram of the VSV genome. le is the leader sequence; tr is the trailer sequence. The 5
genes N, P, M, G, and L were defined in the legend to Fig. 4.1 and are described in more detail in the text. The positions of
the conserved regulatory sequences at the gene boundaries are shown by the triangles. Each of these intergenic sequences
is composed of E (end), I (intergenic), and S (start) domains. (B) Sequences in VSV at the other three gene boundaries.
Data for this figure came from Rose and Schubert (1987).
complementary copy of the genome. The antigenomic RNA
about 1200 molecules of G, present as trimers that form
can be copied by the polymerase activity in the (+)RNP to
spikes visible in the electron microscope, and about 1800
produce more genomic RNA. This also requires that the RNA
molecules of M.
be immediately encapsidated. The new genomic RNP can be
used to amplify the replication of viral RNA or, later in infec-
Vesiculovirus Diseases
tion, can bud to produce progeny virions.
VSV causes nonfatal but economically important and
debilitating disease in cattle, pigs, and horses. The name
Maturation of Virus
of the virus comes from the vesicles that it induces on the
The G protein has a 16-residue N-terminal signal
tongue and lips. These symptoms resemble those caused by
sequence that leads to its insertion into the endoplasmic
foot-and-mouth disease virus and epidemics of VSV disease
reticulum during translation. The signal is removed by
in domestic animals result in disruptive quarantines as well
cellular signalase. The resulting 495-residue protein is
as complications in efforts for control of FMDV. Human
anchored near the C terminus by a 20-residue transmem-
infection is common in rural areas where VSV is endemic in
brane anchor, with the 29 C-terminal residues forming a
domestic animals; 2590% of farmers in such areas may have
cytoplasmic domain (i.e., it is a type 1 integral membrane
anti-VSV antibodies, showing past infection by the virus.
protein). G is glycosylated on two asparagine residues
Human infection is largely asymptomatic or associated with
and transported to the plasma membrane, where progeny
a mild febrile illness, sometimes accompanied by herpes-
viruses are formed by budding (Fig. 2.23D). The M pro-
like lesions in the mouth or on the lips or nose. Serological
tein appears to form an adaptor between the glycoprotein
surverys also show that the virus infects bats, deer, and mon-
present in the plasma membrane and the nucleocapsids
keys in endemic areas. The virus also replicates in numer-
assembled inside the cell. M also acts to repress RNA
ous arthropods and has been isolated from mosquitoes, sand
synthesis by the viral nucleocapsid. The G protein con-
flies, black flies, culicoides, houseflies, and eye gnats. The
tains the fusion activity and receptor recognition activi-
natural cycle of VSV in nature is not understood and the epi-
ties of the virus, and it is the only protein present on
demiological importance of mosquitoes or other hematopha-
the surface of the virion. The assembled virion contains
gous arthropods in transmission of the virus is not clear.
VSV is endemic in Latin America from Mexico to
Rabies Virus
northern South America where outbreaks of disease occur
Most lyssaviruses cause the disease called rabies in
every year. VSNJV accounts for the majority of the clini-
humans and other mammals. It is a uniformly fatal disease
cal cases in this region. Sporadic outbreaks occur both
of man and of other mammals, and has been known since the
north and south of this endemic area. In the United States,
twenty-third century b.c. Rabies virus is present in the saliva
sporadic outbreaks occur in the Southwest at intervals
of a rabid animal and is transmitted by its bite. Infection
of about 10 years, caused by both VSIV and VSNJV.
begins in tissues surrounding the site of the bite. Without
In the Southeast, VSNJV was endemic until the 1970s.
treatment the virus may be transmitted to the brain, where
After this, VSNJV remained endemic only on Ossabaw
replication of the virus leads to the disease called rabies. It is
Island off Georgia, where it is transmitted to feral pigs by
believed that the virus enters neurons by using acetylcholine
sand flies. In the rest of the Southeast, no clinical disease
receptors as a receptor, followed by transport up the axon
caused by VSV has been reported since 1976 and there
until it reaches the cell body. The probability that rabies will
have been only occasional findings of seropositive wild
develop following the bite of a rabid animal depends on the
animals.
location of the bite, the species doing the biting, and the virus
Chandipura virus, another member of the genus
strain. In the absence of treatment, bites on the face and head
Vesiculovirus (Table 4.2), is widespread in India, where
result in rabies in 4080% of cases, whereas bites on the legs
it infects humans and domestic animals. It is also present
result in rabies in 010% of cases. The incubation period to
in Senegal. It has been isolated from sand flies, which are
development of symptomatic rabies can vary from less than
believed to serve as vectors of the virus. Until recently it
a week to several years. Once the virus reaches the brain, it
was thought to cause no disease or only mild febrile illness
spreads from there to a variety of organs. To be transmitted, it
in humans. However, recent epidemics of encephalitis in
must spread to the salivary glands. Infection of neurons in the
children in India in 2003 and 2004 have been traced to the
brain may result in behavioral changes that cause the animal
virus, showing that it has the potential to be a significant
to become belligerent and bite other animals, so that the virus
human pathogen. In the 2003 epidemic in Andhra Pradesh,
present in salivary fluid is transmitted. In humans, the disease
for example, 183 of 329 affected children died.
may be paralytic or may result in nonspecific neurological
More than 20 other vesiculoviruses are known. As one
symptoms including anxiety, agitation, and delirium. Biting
example, Isafahan virus has been isolated from sand flies
behavior is not a consequence of rabies-induced neurological
in Iran. There is serological evidence of human infection in
disease in humans, and human-to-human transmission does
several central Asian countries but no definite evidence for
not occur. Two to 7 days after symptoms of rabies appear,
human illness caused by it.
coma and death ensue. Only three cases of humans recovering
from symptomatic rabies have been recorded.
Genus Lyssavirus
For centuries, the saliva of a rabid dog was thought to
The rhabdovirus of greatest medical interest is rabies
be the source of rabies infection, but it was only in 1804
virus, which belongs to the genus Lyssavirus. Seven geno-
that Zinke succeeded in transmitting rabies from it. In the
types or species of lyssavirus are currently recognized and
late 1800s, Pasteur adapted rabies virus to laboratory ani-
two additional genotypes have been proposed. Genotype
mals and developed the concept of protective vaccination
1 is classical rabies virus and is virtually worldwide in
against rabies. The dessicated spinal cords from rabies-
distribution. It is the only lyssavirus found in the United
infected rabbits became the first rabies vaccine. On July 6,
States where it infects a wide range of hosts, notably
1885, this vaccine was used to immunize Joseph Miester,
raccoons, wolves, skunks, and bats. Mokola virus is an
who had been bitten 14 times by a rabid dog. Because of the
African virus that is known to infect dogs and cats as
multiplicity of bites, he would almost surely have died, but
well as shrews and humans. The remaining five geno-
the Pasteur vaccine saved him. A vaccine grown in nervous
types are bat-associated lyssaviruses. Lagos bat virus and
system tissue and inactivated by phenol rather than drying
Duvenhage virus are African, there are two European bat
was the accepted rabies vaccine for decades. In the 1960s,
lyssaviruses called type 1 and type 2, and Australian bat
a safer inactivated virus vaccine derived from virus grown
lyssavirus is Australian as its name implies. Two addi-
in cultured human cells was introduced. The rabies vaccine
tional genotypes of bat viruses present in central Asia
is unique in that it is normally given after exposure to the
have been proposed.
virus, in conjunction with anti-rabies antiserum. This is pos-
The genome of rabies closely resembles that of the VSV,
sible because there is a window of time following the bite of
although very little sequence identity exists between the
a rabid animal before rabies develops, during which a pro-
genomes of the viruses belonging to the two genera. One
tective immune response can be induced. Veterinarians and
difference is the lack of a second protein encoded in the P
wildlife workers who are potentially exposed to rabid ani-
gene of lyssaviruses.
mals, as well as biologists who work with rabies virus in the
laboratory, are immunized prophylatically, but the protective
protein, using bait containing one of these viruses that is dis-
immune response can be of short duration and immunity
persed by hand or by airplane. In the eastern United States,
must be tested at regular intervals.
spread of baits has been used to slow or prevent the further
In the United States, Canada, and Western Europe, where
spread of rabies up the eastern seaboard. In Europe, baits
vaccination of domestic dogs is widely practiced, wild ani-
have been used to set up barriers to halt the spread of rabies
mals such as raccoons and skunks maintain the virus and
in foxes. The European efforts have been more successful
transmit it to humans or their domestic animals. Fig. 4.3
than those in the United States.
shows the decline in number of cases of rabies in dogs and
The perpetuation of rabies in nature is somewhat of a
humans in the United States since the 1940s, the result of
mystery because of the fact that it can be maintained only
compulsory vaccination of pet dogs. Fig. 4.3 also shows
in rabid animals who die quickly of the infection. How is
the increase in rabies in wild animals since 1940. Fig. 4.4
it that the virus manages to persist? One possibility arises
illustrates the explosive spread of rabies in raccoons on
from recent findings that rabies virus can establish a latent
the eastern seaboard in the last 20 years. The focus of this
infection in humans. Five cases have been documented in
spread in Virginia arose from the import of 3500 raccoons
which people did not develop symptoms for 7 or more years
from Florida into the Washington, D.C., area by members
after infection with the virus. In at least some of these cases,
of the cabinet of President Carter, for the purpose of rac-
progression to rabies appeared to be triggered by hormonal
coon hunting. These imported animals ignited an epidemic
changes during puberty. If the virus can establish a latent
of rabies in raccoons that has slowly spread up and down
infection in other animals that is later followed by reemer-
the Atlantic seaboard, as shown in Fig. 4.4. In other parts of
gence of the virus and its transmission to new susceptibles,
the United States, foxes and skunks are important hosts for
this could serve as a reservoir of the virus.
rabies and disease also occurs at times in wolves and coyo-
Bats may also be an important reservoir of (classical)
tes. Bats are also an important carrier of rabies. In other parts
rabies virus (bat lyssaviruses are discussed separately later).
of the world, where licensing and immunization of pets is
Rabies virus infection of bats seems to take longer to kill
not required, domestic dogs continue to be the principal vec-
the animal, during which time the virus may be transmis-
tors that transmit rabies to humans. Rabies remains a signifi-
sible through the bite of an infected bat or through aerosols
cant global health problem. More than one million people
from infected bat feces or saliva spray. However, rabies virus
annually undergo antirabies treatment following exposure to
in bats is distinguishable from rabies virus strains in other
the virus, and 50,000 people die of rabies each year.
wildlife by nucleotide sequence analysis. Thus, mixing of
Efforts to control rabies in wildlife in the United States and
bat rabies and rabies in other wildlife is infrequent. Bats can
Western Europe have met with some success. These efforts
transmit rabies to humans, and cases of human rabies trans-
involve vaccinating wildlife with attenuated rabies virus or
mitted by bats in the United States have been documented.
with recombinant vaccinia viruses that express the rabies G
In fact, in the United States in recent years, cases of human
60
10,000
Dogs
50
8000
Wildlife
40
6000
30
4000
20
Human Rabies
DEATHS
10
2000
CASES
2010
1940
1950
1960
1970
1980
1990
2000
Year
FIGURE 4.3 Rabies in domestic dogs and wild animals (right scale) versus human cases (left scale) in the United
States 19402003. Note that untreated rabies in humans is uniformly fatal. Data from Smith et al. (1995), and MMWR
Summaries of Notifiable Diseases, 1996 and 2003.
Vermont
Maine
New Hampshire
Massachusetts
New York
Rhode Island
Connecticut
Pennsylvania
New Jersey
Delaware
Ohio
West
Maryland
Virginia
Virginia
Kentucky
North Carolina
Tennessee
South
Carolina
1977-1979
1980-1984
Georgia
1985-1989
Alabama
1990-1993
Florida
1994-1998
1999-2003
No raccoon rabies
detected
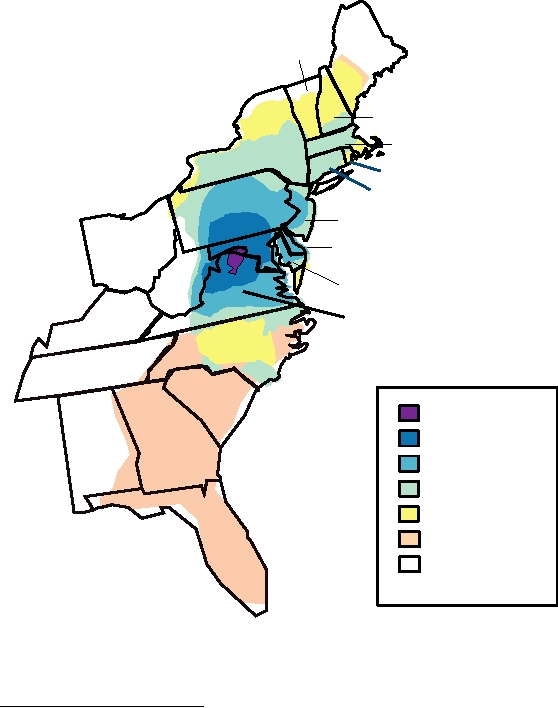
FIGURE 4.4 Spread of raccoon rabies over 3- to 5-year increments in the states of the Atlantic seaboard. Over 25 years
the virus has spread from a small focal area in northern Virginia to encompass much of the entire region from southern
Maine to Florida. From Morbidity and Mortality Weekly Report (MMWR) (1997) Vol. 45, p. 1119, updated with data from
CDC found on www.rabavert.com/caserc.html.
rabies resulting from infection with bat-associated rabies
There are two major types of bats. Bats belonging to the
virus have been more numerous than cases resulting from
suborder Megachiroptera, of which 40 genera are recognized,
infection by bites of other rabid wildlife. In many cases of
are large and feed on fruit and nectar in flowers. Members
bat-associated rabies, the mechanism by which the virus was
belonging to the genus Pteropus are often called flying foxes
transmitted to the human is not known, because no exposure
and are found from Australia across India to Madagascar.
to bats, rabid or otherwise, could be shown.
There are four species of flying foxes in Australia. Bats
belonging to the suborder Microchiroptera are smaller, feed
on insects, and have developed echolocation to find their
Bat Lyssaviruses
prey in the dark. Both types of bats carry ABLV in Australia,
Australia was long believed to be completely free of
and of the two cases of human infection that have resulted,
rabies. However, it has recently been found that many
one was from a flying fox and the second was from an insec-
Australian bats carry a virus known as Australian bat lys-
tivorous bat. The strains of virus in the two types of bats
savirus (ABLV). Two cases of fatal human rabies that were
are distinguishable, differing by about 20% in nucleotide
caused by infection with this bat virus have occurred in the
sequence.
last few years. In one case, a woman caring for injured bats
Two different European bat lyssaviruses exist. Four
was bitten by a bat in her care. In the second incident, a
human deaths resulting from infection by these viruses have
woman was bitten while trying to remove a bat that had
occurred, and there is concern that more cases might occur.
landed on a child. No rabies has been found in other animals,
Mokola virus has also infected humans in Africa.
presumably because there is no efficient mechanism for
transmission of the virus among other mammals present in
Other Genera of Rhabdoviruses
the continent. However, the disease could potentially spread
to dogs and cats that have been introduced into Australia
Bovine ephemeral fever virus, genus Ephemerovirus, is
over the years.
an arbovirus that causes economically important disease in
Search WWH :