The rubella vaccine has now been in use for many years
genera are similar in size (11 kb for flaviviruses, 12.5 kb
and is generally safe and effective when given to children. The
for pestiviruses, 9.4 kb for hepaciviruses) and organization.
present vaccine has a high incidence of side effects in adults,
These viruses, like the picornaviruses, have a genome that
however, especially arthralgia and arthritis. Nonetheless,
contains only a single ORF. This ORF is translated into a
vaccination is recommended for women of childbearing age,
long polyprotein that is processed by cleavage into 10 or
as well as for certain health care personnel, who have never
more polypeptides. Processing of the precursor polyprotein
been vaccinated and who are seronegative. The need exists
is complicated. Cleavage is effected by a combination of
to improve the vaccine, and current efforts to understand the
one or two or three (depending on the virus) virus encoded
molecular biology of the virus in more detail will hopefully
proteases and two or more cellular proteases. The struc-
tural proteins are encoded in the 5′-terminal region of the
lead to the development of a better vaccine.
genome (like picornaviruses). However, all members of the
Flaviviridae are enveloped, unlike the picornaviruses, and
FAMILY FLAVIVIRIDAE
the structural proteins consist of a nucleocapsid protein and
two or three envelope glycoproteins. Cellular proteases make
The Flaviviridae are named after the prototype virus,
the cleavages that separate the glycoproteins, but the cleav-
yellow fever virus, flavus being the Latin word for yellow.
ages in the nonstructural region of the polyprotein, which is
The Flaviviridae are divided into three genera, the genus
required for RNA replication, are made by one or two virus-
Flavivirus, the genus Pestivirus, and the genus Hepacivirus.
encoded proteases. Even so, cellular signalase makes at least
A partial listing of viruses in the three genera is given in
one of the cleavages in the nonstructural domain of flavivi-
Table 3.12. In the following discussion, the term flavivirus
ruses. The cleavage pathways in this genus are described in
refers only to members of the genus Flavivirus unless
detail next.
otherwise specified.
All members of the Flaviviridae encode a serine
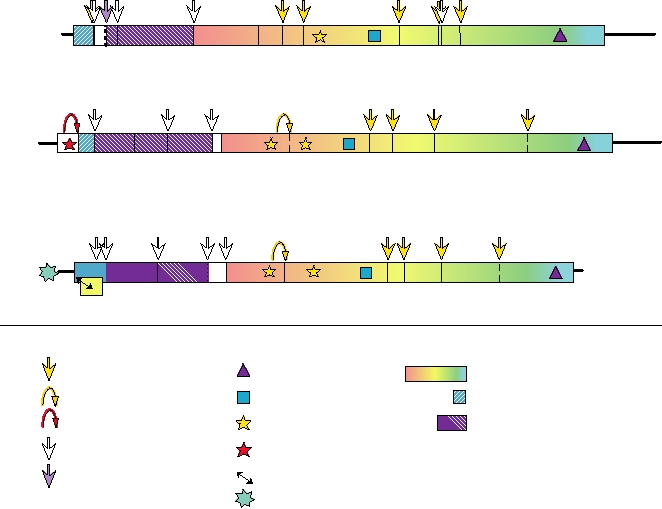
The genome organizations of members of the three
protease with a catalytic triad consisting of serine, histidine,
genera are shown in Fig. 3.27. The genomes of the three
and aspartic acid. The protease resides in the nonstructural
TABLE 3.12 Flaviviridae
Virus name
World
Genus/species
abbreviation
Usual host(s)
Transmission
Disease
distribution
Flavivirus
Dengue (Types 14)
DENV
Humans
Mosquito-borne
Dengue fever, shock,
Worldwide
hemorrhage,
Primatesa
Yellow fever
YFV
Mosquito-borne
Hemorrhage,
Africa,
liver destruction
Americas
Mammals,a
Japanese encephalitis
JEV
Mosquito-borne
Encephalitis
Widespread in
especially swine
Asia
Mammals,a birds
St. Louis encephalitis
SLEV
Mosquito-borne
Encephalitis
North America
Mammals,a birds
Murray Valley
MVEV
Mosquito-borne
Encephalitis
Australia
encephalitis
Mammalsa
Tick-borne encephalitis
TBEV
Tick-borne
Encephalitis
Europe, Asia
Mammals,a birds
West Nile
WNV
Mosquito-borne
Encephalitis
Europe, Africa,
North America
Hepacivirus
Hepatitis C
HCV
Humans
Parenteral,
Hepatitis,
Worldwide
transfusion
liver cancer
Pestivirus
Classical swine fever
CSFV
Swine
Contact
Fever, acute
Europe, Americas
gastroenteritis
Usually noneb
Bovine viral diarrhea
BVDV
Cattle
Contact
Worldwide
a
Including humans.
b
Calves infected in utero develop persistent infections that can lead to mucosal disease.
Flavivirus (Dengue)
?
CAP
C
M
E
NS1
NS2A
NS3
NS4A
NS5
NS2B
NS4B
Pestivirus (BVDV)
?
?
?
E1
E2
NS2-3
NS5A-B
Npro Erns
NS4A
p7
NS4B
NS2
NS3
NS5A
NS5B
C
Hepacivirus (HCV)
E1
E2
NS2
NS3
NS4b
NS5
p7
NS4a
C/F
(p58+p68)
Protease Cleavages
Enzyme Motifs
Coding Domains
NS3 proteinase
Polymerase (GDD)
Nonstructural proteins
NS2-3 autoproteinase
Helicase
Nucleocapsid protein
Npro autoproteinase
Serine proteinase
Virion glycoproteins
Signalase
Papain proteinase
Cellular protease
Ribosomal Frameshift
(furin?)
IRES
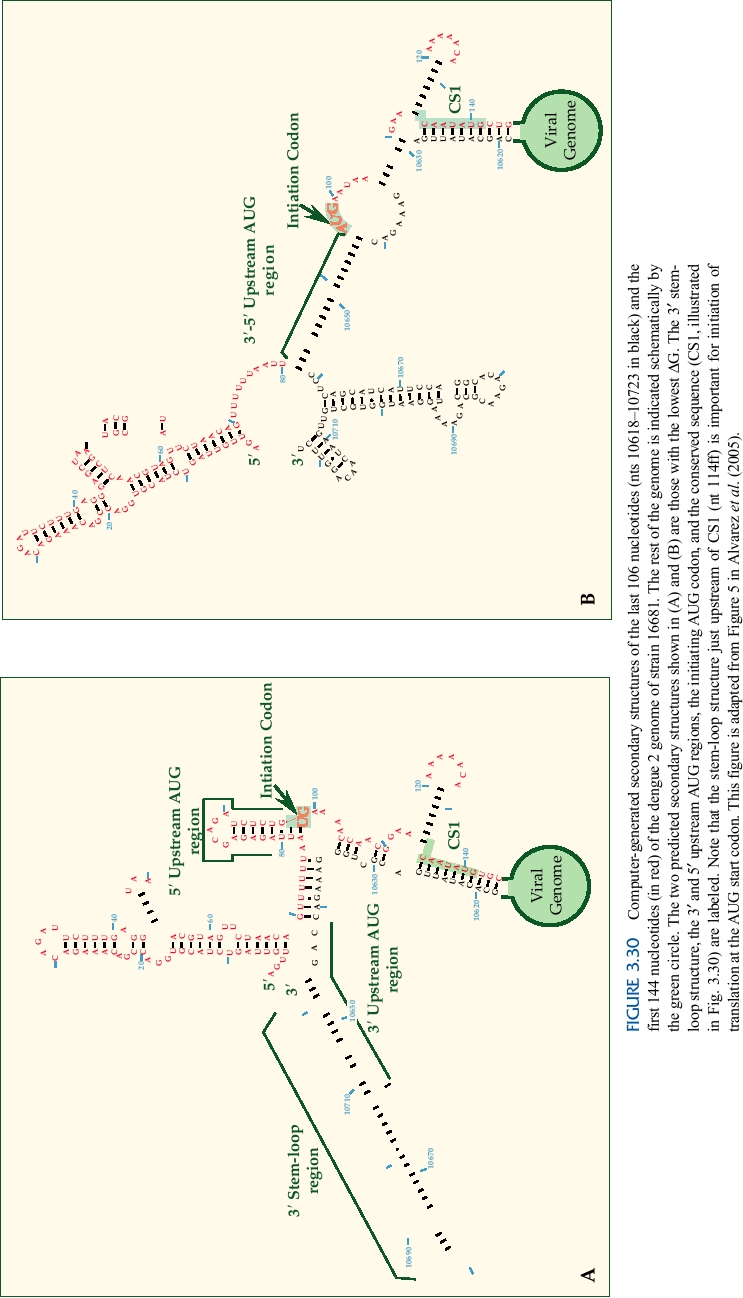
FIGURE 3.27 Genome organization of representatives of the three genera within the Flaviviridae. The cleavage sites
indicated with dashed lines have not been precisely localized. Adapted from Figures 2, 4, 5 of the Flaviviridae in Fauquet
et al. (2005) pp. 981998. The data on the F protein of hepatitis C virus, which is produced from the core protein sequence
by ribosomal frameshifting, came from Xu et al. (2004).
region called NS3, just upstream of a helicase. The crystal
translation, as described in more detail later. No nucleotide
structure of the dengue virus protease and of the hepatitis C
or amino acid sequence identity can be detected between
virus (HCV) protease has been solved to atomic resolution
members of different genera except for isolated motifs that
and they possess a fold similar to chymotrypsin, as is the case
are signatures of various enzymatic functions.
for other viral serine proteases whose structures have been
Viruses in the family are enveloped. As described later
solved. The enzyme is interesting in that a second polypeptide
and in Chapter 2, members of the genus Flavivirus have a
is required for activity, NS2B in flaviviruses and NS4A in
structure that is related to that of alphaviruses. Details of
HCV. From the atomic structure of the HCV protease com-
the structures of pestiviruses and hepaciviruses are lacking.
plexed with the region of NS4A required for activity, it is
Flaviviruses mature at intracytoplasmic membranes rather
clear that NS4A forms an integral part of the folded protease.
than at the plasma membrane.
Thus, it is puzzling that the protease consists of two cleaved
products rather than one continuous polypeptide chain.
Genus Flavivirus
Flaviviruses encode only the NS3 protease. Hepaciviruses
and pestiviruses encode a second protease in the NS2 region that
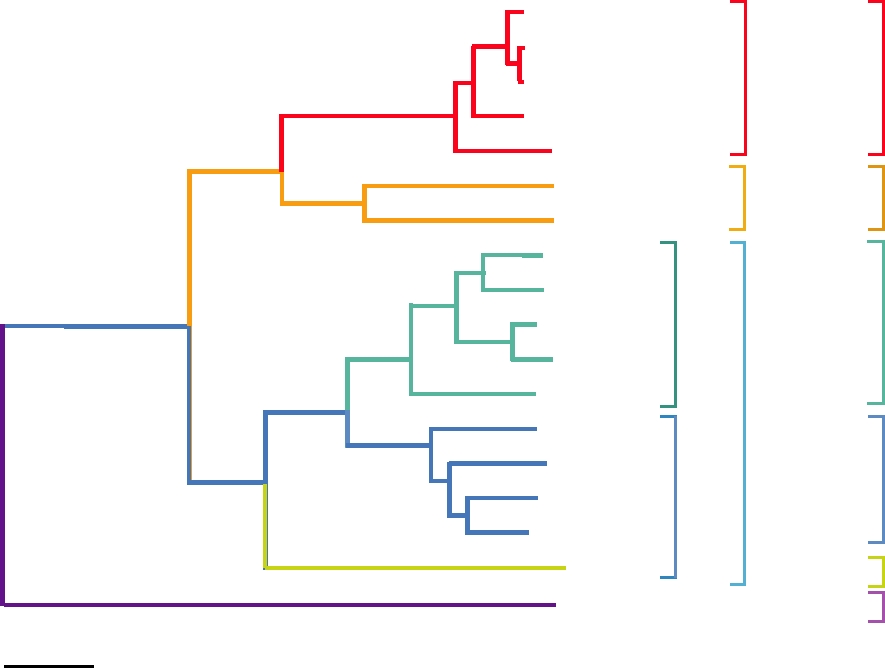
There are about 53 species of flaviviruses currently rec-
cleaves between NS2 and NS3. Pestiviruses also encode a third
ognized, and many species have important subtypes that are
protease at the N terminus of the polyprotein whose only known
also named. A representative sample is given in Tables 3.11
function is to cleave itself from the polyprotein precursor.
and 3.12. The relationships of the viruses to one another is
Flaviviruses have capped genomes whose translation is
illustrated by the dendrogram in Fig. 3.28. All members of
cap dependent. In contrast, the hepacivirus and pestivirus
the genus are closely related and share significant amino acid
genomes are not capped and have an IRES in the 5′ non-
sequence identity in their proteins, which results in serologi-
translated region. Members of family Flaviviridae do not
cal cross-reactivity. Historically, members of this genus were
have a poly(A) tail at the 3′ end of the RNA. Instead, a stable
assigned to it on the basis of these cross-reactions. Most are
stem-loop structure is present at the 3′ end of the genome that
arthropod-borne, and they were once referred to as Group
is required for replication of the genomic RNA and for its
B arboviruses. They can be divided into three major groups
Vector or Host
Serogroup
Louping Ill
TBE (Hypr)
Tick-borne
Tick
TBE (Neu)
Encephalitis
Langat Bat
Powassan
Rodent
APOI
Rio Bravo
Rio Bravo
Bat
JE
Murray Valley
Japanese
Kunjin
Encephalitis
Culex
West Nile
SLE
Mosquito
Dengue 4
Dengue 2
Dengue
Dengue 1
Aedes
Dengue 3
Yellow Fever
Yellow Fever
Cell Fusing Agent
Aedes
CFA
Scale Bar equals distance of 0.13
FIGURE 3.28 Phylogenetic tree of the flaviviruses based on NS3 polyprotein region using the neighbor-joining method.
Data are from Billoir et al. (2000).
based on the vector utilized: the mosquito-borne group (which
introduced into the United States, is vectored by a very wide
includes yellow fever, the dengue complex, and the Japanese
variety of mosquitoes, and this has been in part responsible
encephalitis complex), the tick-borne encephalitis group (the
for the rapid spread of the virus across the United States.
TBE complex), and a group that lacks an arthropod vector.
The last are of limited medical importance. Notice that in
Expression of the Viral Genome
the phylogenetic tree in Fig. 3.28, the tick-borne viruses
and the mosquito-borne viruses belong to different lineages.
The genome organization of a typical flavivirus is illus-
These viruses are adapted to a tick vector or to a mosquito
trated in Fig. 3.27. As for all plus-strand RNA viruses, the
vector, and interchange of vectors does not occur. Further,
genomic RNA is a messenger and in the case of flaviviruses
the tree indicates that the mosquito-borne viruses separate
serves as the messenger for all of the virus encoded proteins.
The RNA is capped but lacks 3′ poly(A). There is a stem-
out into a lineage vectored primarily by mosquitoes belong-
loop structure at the 3′ end which serves the same function
ing to the genus Culex and lineages vectored by mosquitoes
belonging to the genus Aedes. However, in these lineages the
as poly(A) in other messengers. This structure increases the
restriction on the mosquito vector is not firm and mosquitoes
efficiency of translation of the RNA by about 10-fold and
belonging to other genera may vector many of these viruses.
will substitute for poly(A) in model systems. Viral proteins
As two examples, the ancestral yellow fever virus in Africa
are not required for this effect and therefore cellular pro-
is vectored by Aedes mosquitoes in both sylvan and urban
teins must interact with this structure in order to increase the
cycles, but in the Americas it is vectored by Hemagogous
efficiency of translation. It is known that during translation
mosquitoes in a sylvan cycle and Aedes mosquitoes in an
of mRNAs that are capped and polyadenylated, there is an
urban cycle, as described later. West Nile virus, recently
initiation complex formed that contains both cap-binding
protein and poly(A)-binding protein. Thus, the complex
nal signal sequences is responsible for the multiple insertion
interacts with both ends of the mRNA to initiate translation.
events required to insert prM, E, and the following protein,
It is assumed that a cellular protein binds the 3′ stem-loop of
NS1, into the endoplasmic reticulum. After separation of
flaviviruses and interacts with the initiation complex so as to
these three proteins by signalase, prM and E form a het-
perform the same function as the poly(A)-binding protein.
erodimer. prM is cleaved to M by furin during transport of
Formation of this complex in the case of flavivirus RNAs
the heterodimer or during virus assembly. Assembly of viri-
could be enhanced by cyclization of the viral RNA described
ons is described in more detail later.
later, although the primary function of cyclization appears to
Following the E protein is NS1 (NS for nonstructural).
be in the replication of the viral RNA.
NS1 is a glycoprotein and has multiple functions that are
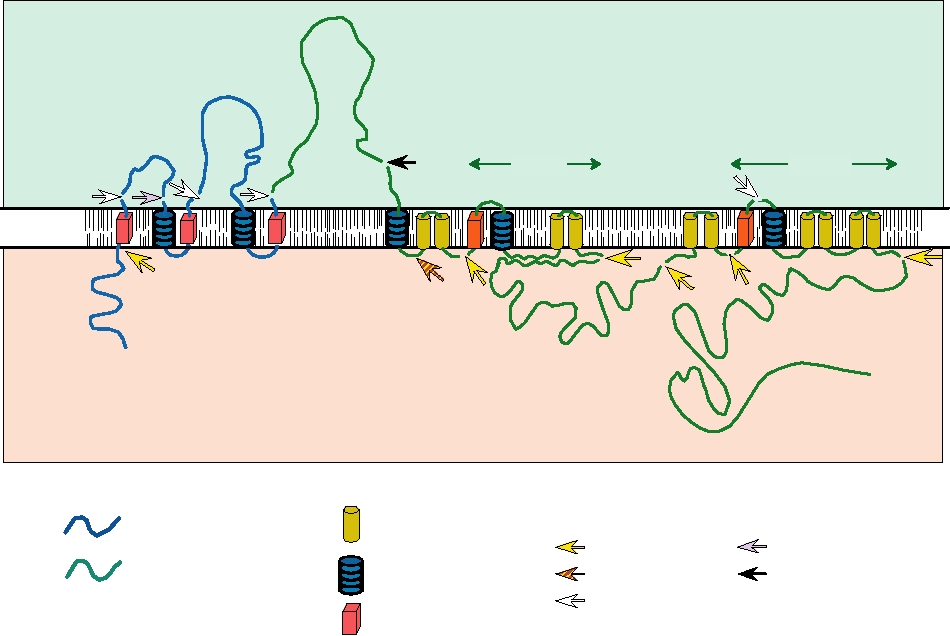
The processing of the long polyprotein produced from
only poorly understood. It is found as dimers and higher
the genome is complicated and is illustrated in Fig. 3.29 as
multimers in three locations in mammalian cells: intrac-
an example of complex processing events that can occur in
ellular; anchored in the plasma membrane by a GPI (gly-
viral polyproteins associated with lipid bilayers. The nucleo-
cosyl-phosphotidylinositol) anchor; and as a soluble protein
capsid protein is 5′ terminal in the genome and is removed
secreted from the infected cell. It is required for RNA rep-
from the precursor polyprotein by the viral NS2BNS3 pro-
lication, presumably a function of the intracellular form of
tease. Two envelope proteins, prM (precursor to M) and E
the protein. For this function, it interacts with NS4A. The
(envelope), follow. Both are anchored in the endoplasmic
cell surface-anchored form is capable of antibody-induced
reticulum by C-terminal membrane-spanning domains and
signal transduction that may play a role in cell activation.
are usually, but not always, glycoproteins. A series of inter-
The function of the secreted form is unknown but it has
NS1
E
prM
NS4B
NS2B
N
N
N
Lumen
NS2A
NS4A
ER membrane
Cytoplasm
N
N
C
NH3+
NS5
NS3
COOH
Intramembrane
Proteolytic Cleavages
Structural proteins
domain
Golgi protease (Furin?)
NS2B-3
unknown protease
Stop transfer
Nonstructural proteins
NS2B-3 (alternative)
signal
Signalase
Signal
sequence
FIGURE 3.29 Processing of the flavivirus polyprotein into the structural and nonstructural proteins of the virus. The
structural proteins (blue) at the N terminus of the polyprotein are processed primarily by signalase, with one late cleavage in
prM due to furin. The nonstructural proteins (green) are mostly processed by the viral NS2BNS3 protease. As indicated in
the figure, the central 40 amino acids of NS2B interact with NS3, tying NS3 to the membrane, and this interaction is essential
for proteolytic function. The striped arrow shows the alternative site of cleavage within NS2A that may lead to an anchored
form of NS1. Adapted from Figures 3, 4, and 6 in Strauss and Strauss (1996).
been speculated that it has a role in counteracting immune
cyclize the RNA are illustrated in Fig. 3.30, where two possi-
responses to the virus.
ble structures are shown. Experimental data have shown that
Next in the polyprotein precursor are two hydrophobic
the RNA sequence in the capsid protein downstream of the
polypeptides called NS2A and NS2B. These proteins are
AUG start codon is involved in cyclization (region marked
cleaved by the viral NS2BNS3 protease. They are associ-
CS1). Sequences upstream of the start codon are also known
ated with membranes and may serve to anchor parts of the
to be required for cyclization, and the two structures show
replication machinery to internal membranes in the cell.
different ways that these might be used for cyclization. This
figure also illustrates the long stem-loop structure at the 3′
NS2A has multiple functions. It inhibits the production of
interferon-α /β by infected cells. As described in more detail
end of the RNA, discussed earlier. A stem-loop structure in
the 5′ region just upstream of the CS1 region has also been
in Chapter 10, the interferons are potent inhibitors of virus
replication and most, perhaps all, viruses encode products
shown to be important in translation of the RNA, in this case
to block interferon action. NS2A also has a role in the pro-
for recognition of the AUG start codon, which is found in a
duction of infectious particles from the infected cell, since
poor context for a start codon.
certain mutations in this protein block virus assembly but do
The sequences surrounding CS1 are illustrated for a
not affect other aspects of the virus life cycle. These mutants
number of mosquito-borne flaviviruses in Fig. 3.31. This
can be suppressed by changes in the NS3 helicase domain,
eight nucleotide sequence is invariant among the mosquito-
suggesting an interaction between NS2A and NS3. NS2B
borne flaviviruses, and experimental studies have shown
also interacts with NS3, but with the protease domain. It
that this sequence is important for cyclization and replica-
tion of the RNA. The 3′ sequences complementary to this
is a cofactor required for the NS3 protease activity and the
region are found in the 3′ nontranslated region (see also Fig.
central domain of NS2B forms a complex with NS3, which
follows NS2B in the polyprotein precursor. The NS2BNS3
3.30). Changes in these sequences that eliminate cyclization
serine protease cleaves many bonds in the polyprotein. NS3
prevent the RNA from replicating, even in model systems
also has at least two other activities--the middle domain of
in which translation of the RNA is not required for expres-
NS3 is a helicase, required for RNA replication, and the C-
sion of the replicase. Compensating mutations in the partner
terminal domain has RNA triphosphatase activity, an activ-
sequence that restore cyclization restore the ability of the
ity required for the capping of the viral genome.
RNA to replicate. Thus, cyclization is required for RNA rep-
NS4A and NS4B are hydrophobic polypeptides that are
lication.
associated with membranes. They may function in assembly
The identities of the promoters recognized by the RNA
of the viral replicase on intracellular membranes. Both the
replication machinery are as yet unknown, but the require-
viral NS2BNS3 protease and cellular signalase are required
ment for cyclization suggests that sequences at both ends of
to produce the final cleaved products.
the RNA are required. The conservation of the 8-nucleotide
NS5 is the viral RNA polymerase. It appears to be a
core sequence suggests that these sequences might be part of
soluble cytoplasmic protein that associates with membranes
the promoter recognized by the RNA replicase.
through association with other viral peptides. It also has
methyltransferase activity and thus is the capping enzyme
Formation of the Virion
that caps the viral genome. Thus, capping requires two fla-
viviral proteins, NS3 (RNA triphosphatase) and NS5 (cap-
Most flaviviruses mature at intracellular membranes.
ping enzyme). Note the similiarity to alphaviruses where the
Budding figures have been described only rarely and assem-
RNA triphosphatase activity is also on the helicase-protease
bly may be associated with the complex processing of the
protein (nsP2, the analogue of flaviviral NS3), and the meth-
polyprotein. West Nile virus is an exception to this general
yltransferase or capping activity is a different protein (nsP1).
rule. It grows to higher titers in cultured cells than other fla-
However, in alphaviruses the capping enzyme and the RNA
viviruses and budding of preassembled nucleocapsids at the
polymerase (nsP4) are distinct proteins, whereas in flavivi-
plama membrane is readily seen. Even in this case, however,
ruses they are present in the same polypeptide.
intracellular assembly of virions is also seen.
The processing of the structural proteins from the precur-
sor polyprotein was described earlier. prM and E form a het-
Replication of the Viral RNA
erodimer shortly after synthesis. The assembly of flaviviruses
RNA replication is associated with the nuclear mem-
has clear parallels with that of alphaviruses, as described in
brane. The composition of the replicase complex is not
Chapter 2. E of flaviviruses and E1 of alphaviruses are
understood but is assumed to consist of many (most? all?)
homologous proteins, having the same structure and func-
of the viral nonstructural proteins with associated cellular
tion (see Fig. 2.17). A heterodimer is first formed, between
proteins. Cyclization of the RNA is required for replication.
prM and E in flaviviruses and PE2 and E1 in alphaviruses.
Sequences from the 5′ and 3′ regions of dengue virus RNA
Immature virus particles can be isolated that have uncleaved
that form a number of stem-loop structures and that also
PE2 or prM whose infectivity is very low. The trimeric spikes
G
C C
G G
C G
C A
U A
U A
U
G
C C
G
A
C
A
U C
G C
G
G
C U
A C
G U
A C
G U
A A
U
C C G A
U C
G G
C A
U
G
G
A
U G
C A
U
C G
C
G
C G
C G
A
U A
U A
U
G
C C
G U
A C
G
A
U G
C A
U
U
U
C
G C
G U
A G
C
C
A
U
A GU
C
C
U
AA U
CC
G
U
U
C
UG A
U G
C C
G A
U U
G C
G A
U U
A U
A C
G C
G A
AU
A
A
G
A
G AC G
C CA
G
C
C
A
A
AG
CS1
5(
A
3
147) C C C UGGGC G UC AAUAUG GUAC GAC GAG (173)
YF
(126) CC CC GGGUCG UC AAUAUG C UAA AAC GC G (153)
MVE
(126) AA CC GGGC UA UC AAUAUG C UGAA AC GC G (153)
JE
(127) AA CC GGGC UG UC AAUAUG C UAA AAC GC G (154)
WN
(129) AA CC GGGUUG UC AAUAUG C UAA AAC GC G (156)
SLE
(127) GA CC AC CU U UC AAUAUG C UGAAAC GC G (153)
DEN4
(125) C AC GC CU U UC AAUAUG C UGAAAC GC G (151)
DEN2
FIGURE 3.31 Conserved nucleotide sequence elements in the 5′ region encoding the capsid protein in six different
mosquito-borne flaviviruses. The number of the first and last nucleotides shown is given in parentheses. The boxed nucleotides
in red are those postulated to be important for cyclization of the RNA. Residues shaded in green are complementary to
sequences at the 3′ end and those given in blue are conserved but probably not involved in cyclization. Adapted from Figure
7 of Hahn et al. (1987).
in these immature particles are quite similar in structure (see
by fever and rash. Several important viruses and their dis-
Fig. 2.15). Flaviviruses have a triangulation number of 3 and
eases are listed in Tables 3.11 and 3.12. We begin the discus-
therefore 60 trimeric spikes each consisting of 3 heterodimers
sion of these viruses with yellow fever virus, the prototype
of prM and E. Alphaviruses have a triangulation number of 4
flavivirus and a virus whose history was important in the
and therefore there are 80 trimeric spikes each consisting of
development of the science of virology and of vaccinology.
3 heterodimers of PE2 and E1. After cleavage of prM or of
Yellow fever virus (YFV) was once greatly feared and is
PE2, however, the structures are quite different. Alphaviruses
still capable of causing large epidemics. The virus is viscero-
retain 80 trimeric spikes with E2-E1 heterodimers. In flavivi-
tropic in primates, the only natural hosts for it. The growth
ruses, however, the M-E heterodimer dissociates and there is
of the virus in the liver, a major target organ, causes the
a dramatic rearrangement whereby 90 E-E homodimers are
major symptoms of disease and the symptoms from which
formed and the particle shrinks from 60 nm in diameter to
the name of the virus derives, jaundice following destruc-
50 nm. Upon infection of a cell and exposure of the mature
tion of liver cells. The virus also replicates in other organs,
flavivirion to acidic pH there is another dramatic rearrange-
such as kidney and heart, and causes hemorrhaging. Illness
ment and 60 E-E-E homotrimers are formed that tilt up so
is accompanied by high fever. Death occurs in 2050% of
that the fusion peptide at the extremity of domain 2 is inserted
serious infections, usually on days 710 of illness and usu-
into the cellular target membrane and fusion results. It seems
ally as a result of extensive liver necrosis.
clear that the structures of alphaviruses and flaviviruses had a
YFV is present today in Africa and Latin America.
common origin, and that recombination during their evolution
It originated in Africa and spread to the Americas with
resulted in this common structure becoming associated with
European colonization and the introduction of slaves. The
different suites of RNA replication enzymes. It is possible that
virus is maintained in two different cycles. In an endemic or
this structure, which allows enveloped viruses to have a regu-
sylvan cycle, it is maintained in Aedes africanus and other
lar icosahedral structure, evolved only once. It is also of note
Aedes mosquitoes in Africa and in Haemogogus mosquitoes
that both groups of these viruses are primarily arboviruses and
in the Americas. Monkeys form the vertebrate reservoir. In
perhaps this common structure is important for this.
this cycle, forest workers and other humans who enter deep
The flavivirus nucleocapsid is thought to be icosahedral
forests are at risk. Infection of humans can lead to the estab-
in symmetry, perhaps having a triangulation number of 3.
lishment of an epidemic or urban cycle in which the virus
There appears to be no interaction between the envelope
is transmitted by the mosquito Aedes aegypti and humans
proteins and the capsid proteins in flaviviruses, however,
are the vertebrate reservoir. In this cycle, all urban dwellers
unlike the situation for alphaviruses, so that the icosahedral
are at risk. Aedes aegypti is a commensal of man, breed-
structure of the nucleocapsid, if it exists, is not coordinated
ing around human habitation. It is widespread in the warmer
with the icosahedral arrangement of the glycoproteins form-
regions of the world, including the southern United States,
ing the outer surface of the virion.
Central America and the Caribbean, large regions of South
America, sub-Saharan Africa, the Indian subcontinent,
Southeast Asia, Indonesia, and northern Australia.
Yellow Fever Virus
History of Yellow Fever
Many flaviviruses are important pathogens of humans.
Different viruses may cause encephalitis, hemorrhagic fever
In the 1800s, YFV was continuously epidemic in the
with shock, fulminant liver failure, or disease characterized
Caribbean region, where it had a pronounced influence on
the development and settlement of the Americas by the
there was no disease aboard, the ship was allowed to dock.
Europeans. Caucasians and Native Americans are very sen-
Yellow fever soon appeared in Norfolk. A number of early
sitive to yellow fever, usually suffering a serious illness
cases among the citizens of the town were ascribed to the
with a high death rate. Black Africans, who were brought
ship passing within a half mile of their homes, and it is pos-
as slaves to the New World to replace Native American
sible that infected mosquitoes were blown ashore, although
slaves who had died in large numbers from European dis-
it is also possible that workmen visiting the ship while laid
eases, in general suffer less severe disease following yellow
up for repairs may have brought the disease into the town.
fever infection, presumably having been selected for partial
The disease then spread in all directions at a uniform rate of
resistence by millennia of coexistence with the virus. Their
about 40 yards per day until it encompassed the whole city.
relative resistance to yellow fever resulted in the importa-
The epidemic peaked at the end of August and died out after
tion of even more black slaves into yellow fever zones. The
a frost in October. During the epidemic, an estimated 10,000
high death rate among French soldiers sent to the Caribbean
cases of yellow fever occurred in a population of 16,000, and
region to control black slaves was probably responsible for
2000 died of the disease. The report established two other
the decision by Napoleon to abandon the Louisiana territory
facts about the disease: Persons who had had yellow fever
by selling it to the United States, by which the United States
previously were immune, and the epidemic was not spread
underwent a huge territorial expansion. The high death rate
by person-to-person contact.
among French engineers and workers in the 1880s under de
The Walter Reed Investigation
Lesseps, who had previously supervised the construction of
the Suez Canal, led to the abandonment of the attempt by the
At the turn of the twentieth century, there was much
French to build a canal through Panama. The Panama Canal
debate as to the mechanism by which yellow fever spread.
through Panama was built by the United States only after
The Department of the Army sent an expedition, under the
yellow fever was controlled.
command of Walter Reed, to Cuba, recently acquired by the
From its focus in the Caribbean, yellow fever regularly
United States from Spain, to study the disease. The commis-
spread to port cities in the southern and southeastern United
sion undertook to test the thesis that the virus was transmit-
States and as far north as Philadelphia, New York, and
ted by mosquitoes, using themselves as human volunteers.
Boston. Epidemic yellow fever even reached London. The
Mosquitoes were allowed to feed on yellow fever patients
virus also spread up the Mississippi River from New Orleans.
and then on members of the commission. At first there was
The virus was transported from its focus in the Caribbean by
a lack of understanding about the fact that mosquitoes are
ships, which carried freshwater in which mosquitoes could
infected only by feeding on patients early in their disease,
breed. If there was yellow fever on the ship, the disease was
before an effective immune response arises, and about the
maintained and could be transmitted by the mosquitoes or
necessity for an extrinsic incubation period in the mosquito,
by infected individuals to ports at which the ships called.
during which the virus establishes an infection in the salivary
Yellow fever epidemics could afflict most of the population
glands, before it can transmit the virus. Ultimately, however,
of a city and result in death rates of 20% or more of the city's
the investigation team did succeed in infecting themselves
original population.
by mosquito transmission and one member of the commis-
One telling account of an epidemic in Norfolk, Virginia,
sion, Dr. Jesse Lazear, died of it. Fortunately, his was the
in 1855 is described in the report of a committee of phy-
only death recorded in these experiments. It is of note that in
sicians established to examine the causes of this epidemic.
the days before the introduction of a vaccine, most research-
Quarantine procedures to prevent the introduction of yellow
ers who studied yellow fever in the field or in the laboratory
fever were often thwarted by captains who concealed the
ultimately contracted the disease and many of them died.
presence of the disease to avoid a lengthy quarantine, even
With the discovery that the virus was mosquito borne, the
going to the extreme of secretly burying crew members who
U.S. Army began a campaign in Havana to eliminate mos-
died while in quarantine. On June 6, 1855, the steamer Ben
quito breeding places by eliminating sources of water around
Franklin arrived from St. Thomas and anchored at the quar-
human habitation. It was (and still is) common for drinking
antine ground. There had been three cases of yellow fever on
water to be stored around houses throughout Latin America
the ship during the voyage, of whom two died and were bur-
in large pots that served as excellent breeding places for
ied, one on land and one at sea. There was yet another case
Ae. aegypti. The campaign, which included smashing such
on board during quarantine who died and was buried ashore.
water containers, succeeded in breaking the mosquito trans-
Yet when the health officer, Dr. Gordon, visited the ship, he
mission cycle and yellow fever as an epidemic agent dis-
was told by the captain that there was no disease on the ship.
appeared from Havana within months. This approach was
The captain did admit that there had been two deaths dur-
later exported to other areas with great success, including
ing the voyage but ascribed them to other causes. After 13
Panama. These successes led to the belief that yellow fever
days in quarantine and continued inspection by Dr. Gordon,
could be eradicated, but the discovery of the endemic cycle
who finding nothing amiss believed the captain's report that
of yellow fever dispelled this idea. Forest workers who cut
down trees and brought the mosquitoes down from the upper
spread of epidemic yellow fever in Latin America and, with
canopy, where they transmit the disease to monkeys, were
less success, in Africa. The success of this vaccine has served
particularly at risk. Once infected, a person is able to bring
as a model for the development of other live virus vaccines,
the disease back to town where it can get into the Ae. aegypti
namely, passing the virus in cultured cells from a nonnative
population and start an urban epidemic.
host. Recent sequencing studies have found that the 17D vac-
cine differs from the parental Asibi strain at 48 nucleotides
The Yellow Fever Vaccine
that result in 22 amino acid substitutions. The substitutions
In the late 1920s, yellow fever virus was successfully prop-
responsible for the attenuation of the virus are not known, but
agated in rhesus monkeys, in which it causes a lethal disease
it is suggestive that 8 of the amino acid substitutions are found
and in which it can be experimentally passed from monkey to
in the E protein, where they might alter host range.
monkey. One such strain was derived from an infected human
Yellow Fever Today
named Asibi. Theiler and Smith passed the Asibi strain of
yellow fever in chicken cells, and after approximately 100
Although not as wide ranging as previously, yellow fever
passages, it was found that the resulting virus was no longer
continues to cause epidemics in Africa and South America as
virulent for rhesus monkeys. After additional passages, this
illustrated in Fig. 3.32. On an annual basis, 50300 cases are
virus, called 17D, was ultimately used as a live virus vac-
officially reported in South America and up to 5000 cases in
cine in humans and has proved to be one of the best and most
Africa, but these figures are significantly underreported
efficacious vaccines ever developed. The vaccine virus has
and the World Health Organization estimates that there are
been given to about 350 million people. It causes very few
200,000 cases of yellow fever each year with 30,000 deaths.
side reactions, although three recent vacinees developed full-
Between 1986 and 1991, annual outbreaks of yellow fever
blown yellow fever and died. The vaccine is essentially 100%
occurred in Nigeria that probably resulted in hundreds of
effective in providing long-lasting protection against yellow
thousands of cases. An intense campaign beginning in 1992
fever. This vaccine is routinely given to travelers to regions
to vaccinate the population of Nigeria has resulted in the vir-
where yellow fever is endemic and is used to control the
tual disappearance of yellow fever in Nigeria, but epidemics
66
10
182
1
150
309
74
4
15
11
1540
2
824
97
72
815 251 382 262
1246
1239
340
260
46
325
44
Cases of Yellow
Fever 1992-2004
0
1-10
11-100
101-1000
1000-10,000
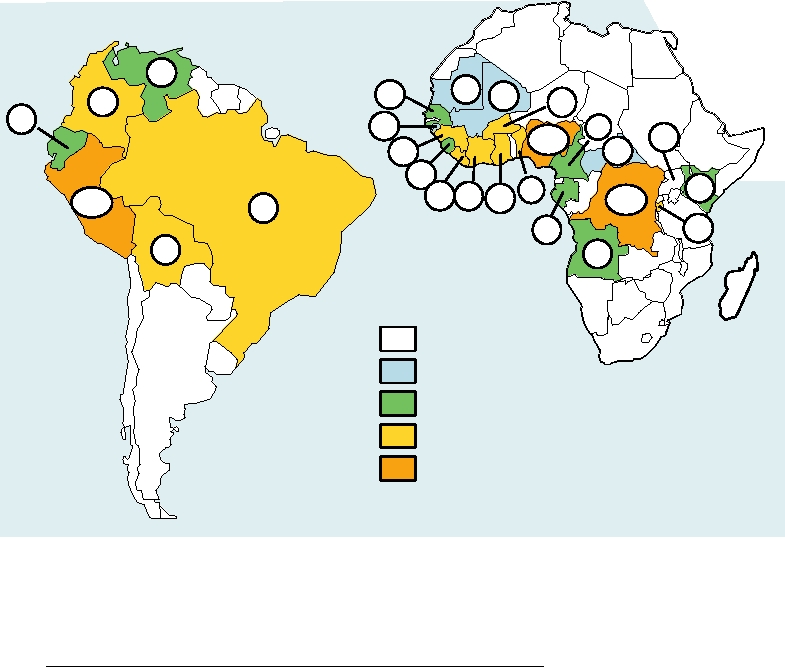
FIGURE 3.32 Cumulative number of cases of yellow fever reported to the World Health Organization for the years 1992
through 2004, by country. It is suspected that cases in Africa may be underreported by a factor of 10 or more. Immunization
coverage in Africa has remained low and the disease has continued to spread. Major epidemics (>250 cases) have occurred
in Liberia, Burundi, and Peru in 1995, Guinea in 2000, Burkina Faso in 2002, and the Democratic Republic of Congo in
2004. Note that while Nigeria had 19,891 cases between 1980 and 1991, since 1994 there have been only 12 cases. Data
from: http://www.who.int/immunization_monitoring/data/data_subject/en/index.html.
continue to occur in other African countries. Epidemics of
of infected cells results in increased cytokine production and
yellow fever also continue to occur in Peru, Bolivia, Brazil,
can result in capillary leakage and shock. Although second
Ecuador, Columbia, and Venezuela, perhaps in part due to the
infections are common, infection by a third serotype is rare.
reemergence of Ae. aegypti in South America as described
Evidently the boost to the immune system from the second
in more detail later. There was one imported case of yellow
infection results in an increase in the amount and avidity of
fever in the United States in 1996, in which an American who
cross-reactive antibodies.
visited the jungles of Brazil along the Amazon River without
Although infection by a second serotype is important for
being immunized returned to the United States with yellow
the development of DHF, it is also known that the probability
fever and died of the disease. Because of the endemic cycle
of contracting DHF is in part a function of the virulence of
in which monkeys are the reservoir, it is probably impossible
the virus that is responsible for the second infection. Some
to eradicate the virus as has been done with smallpox and as
dengue strains grow better than others and are more likely to
is planned for poliovirus and measles virus.
cause DHF upon a second infection than other strains of the
same virus. As one example, there was very little DHF in Sri
Lanka before 1989 despite the continuing circulation of all
Dengue Viruses
four dengue serotypes. In 1989, however, a new strain of
The four dengue viruses, now considered by the ICTV to
DEN-3 appeared in Sri Lanka that caused a large number of
be serotypes of a single viral species, have recently undergone
cases of DHF. A second example, DEN-2 in the Americas,
a dramatic expansion in range. The incidence of dengue fever
is described in Chapter 8.
is estimated to have increased 30-fold over the last 40 years
Because second infections by a different serotype are
and dengue viruses now infect an estimated 50100 million
much more likely to lead to DHF than primary infections,
humans each year. Infection may be subclinical or may result
the development of vaccines against dengue has progressed
in dengue fever, which is usually uncomplicated but which
slowly. The possibility is real that immunizing against one
can progress to dengue hemorrhagic fever (DHF) or den-
serotype might put a person at risk for a more serious ill-
gue shock syndrome (DSS). Uncomplicated dengue fever is
ness. Current efforts in Thailand are directed toward devel-
characterized by headache, fever, rash, myalgia (muscle pain,
oping a quadrivalent attenuated virus vaccine that would
from myo = muscle and algia = pain), bone pain, and prostra-
immunize against all four serotypes simultaneously. U.S.
tion. The disease may be mild or it may be extremely pain-
scientists are independently attempting to develop vaccines
ful (an old name for the disease is break-bone fever which
for the viruses, based either on attenuated dengue viruses
dramatically describes the joint pain that can occur), but it is
or on the development of chimeric flaviviruses that express
almost never fatal. However, progression to DHF or DSS is
dengue envelope antigens in a yellow fever vaccine back-
associated with a significant mortality rate. In the absence of
ground (see Chapter 11). These various vaccine candidates
medical care, mortality can be as high as 20%, but with good
are in clinical trials as of this writing. A major problem has
medical care the mortality rate is a few percent. Up to 250,000
been the tendency of vaccinated humans to respond strongly
cases of DHF and DSS are recorded each year, most of them
to one of the four serotypes in live virus vaccines, often to
in children, and in Southeast Asia DHF and DSS are a lead-
DEN-3, while responding only weakly or not at all to other
ing cause of mortality in children. DHF and DSS have also
serotypes. Changing the ratios of the four viruses in the mix
become important in Latin America. It is thought that DHF
and use of multiple inoculations are being tested as possible
and DSS are caused by immune enhancement in which infec-
ways to overcome this problem.
tion by one serotype of dengue virus expands the population
Dengue viruses are maintained in Ae. aegypti in urban set-
of cells that can be infected by a second serotype. Many anti-
tings in most of the world, but also in Ae. albopictus in Asia,
bodies induced by the four dengue viruses are cross-reactive,
and humans are the vertebrate reservoir. Part of the difficulty
reacting not only with the infecting virus but with the other
in developing vaccines is that there is no animal model for
dengue viruses as well. Immediately following infection,
the disease. The virus will infect monkeys but does not cause
in fact, a person is immune to all four serotypes. With time
disease in them. Small animal models of infection exist but
this cross protection fades, and in less than a year the person
the infection process is artificial and the resulting disease is
remains immune only to the infecting virus, probably for life.
not dengue fever (DF) or DHF. DF and DHF are exclusively
After this time, infection with another serotype can occur and
human diseases and the dengue viruses that infect humans
cause disease. Still present, however, are cross-reactive anti-
are exclusively human viruses.
bodies that can react with the newly infecting virus but which
The Spread of Dengue Viruses
cannot neutralize the virus to provide protection. The nonneu-
tralizing antibodies are thought to enable the virus to infect a
The four serotypes of the dengue viruses arose in Old
larger number of lymphocytes by means of Fc receptors (see
World monkeys and jumped to humans an estimated 200
Chapter 10) than would otherwise result from infection only
1000 years ago. As noted before, the human viruses are now
of cells expressing the dengue receptor. This expanded pool
strictly human viruses. Although they will infect monkeys
under laboratory conditions (without causing disease), the res-
ease is greatly underreported. Only one JE virus infection
ervoir in nature is exclusively humans. However, the monkey
in 200 or 300 results in encephalitis, with children and the
viruses still exist as monkey viruses in a sylvatic cycle in Asia
elderly being at higher risk. The fatality rate following JE
and Africa. The human viruses have been continuously active
encephalitis is 240% in different outbreaks, but 4570%
over large areas of Asia and the Pacific region for many years.
of survivors have neurological sequelae. In endemic areas,
In areas of Thailand where the viruses are endemic, for exam-
virtually all people have been infected by the time they reach
ple, most people are infected by multiple serotypes in child-
adulthood. Birdmosquitobird transmission is the normal
hood and DHF is a leading cause of mortality in children.
transmission cycle, but domestic pigs are particularly impor-
The viruses have recently dramatically expanded their
tant amplifying hosts for transmission to humans because
range in the Americas. Before 1970 there was very little
they are found in proximity to their human owners. Various
dengue activity in the Americas, probably because of mos-
species of Culex mosquitoes transmit the virus. During
quito control efforts that were abandoned about that time.
peak transmission seasons, up to 1% of Culex mosquitoes
Following this, dengue activity increased dramatically, espe-
around human habitations may be virus infected. Travelers
to endemic regions have a probability of about 10-4/week
cially upon the introduction of new strains of the virus from
Asia. By the 1990s there occurred widespread epidemics
of contracting JE, and 24 cases of JE encephalitis in travel-
that affected many millions of people every year. Epidemics
ers were reported between 1978 and 1992. Inactivated virus
have resulted in an estimated 100 million cases of dengue
vaccines are in use in different regions of Asia. The Japanese
infection in Brazil alone. The introductions of Asian viruses
have long used such a vaccine to eliminate JE encephalitis
included more virulent strains of dengue that together with
from their population, and the Chinese have recently devel-
the circulation of multiple serotypes led to epidemics of
oped a vaccine that is being used in China and Thailand.
DHF. Dengue virus is now a major health problem in Latin
The Japanese vaccine is available in the United States for
America. Dengue has also become more active in the Pacific
travelers to endemic regions. Of considerable interest is the
region. Recent epidemics in Hawaii, the first in 50 years,
finding that JE virus infection may reactivate in mice after
have resulted in more than a hundred documented cases of
the immune system first damps it out. Reactivation in other
dengue fever. This topic of the origin and spread of dengue
animals may also occur and could be important for persis-
to the Americas is discussed in more detail in Chapter 8.
tence of the virus in nature.
For the dengue viruses, immune enhancement is impor-
tant for the disease caused in humans. There is no evidence
Japanese Encephalitis Virus
that immune enhancement plays a role in the disease caused
The Japanese encephalitis (JE) complex of flaviviruses
by JE virus or other flaviviruses such as MVE virus. It is
includes a large number of related viruses, many of which
interesting, however, that in a mouse model system, prior
cause encephalitis in humans. For these viruses, the majority
treatment with subneutralizing concentrations of anti-JE
of human infections are inapparent and, for most, fewer than
serum resulted in an increase in virus growth and in mortal-
1% of infections result in neurological disease. However,
ity in the mice following infection by MVE virus. This sug-
when encephalitis develops it is often serious with case
gests that the potential for immune enhancement exists for
fatality rates as high as 50% and neurological sequelae are
other flaviviruses but does not occur in humans other than
frequent among survivors. In addition to JE, these include
the dengue viruses because the immune reaction to flavivi-
St. Louis encephalitis (SLE), Murray Valley encephali-
ruses is normally strong and not cross-reactive and subneu-
tis (MVE), and West Nile viruses. The close relationships
tralizing concentrations of antiviral antibodies do not exist.
of these viruses are illustrated in Fig. 3.28. Some of these
It does raise a warning flag for vaccines, however, and vac-
viruses are widespread whereas others are much more local
cines that increase the seriousness of disease caused by sub-
in their distribution. West Nile virus, for example, is now vir-
sequent infection have occurred in the case of measles virus
tually worldwide (the Australian strain is often called Kunjin
and respiratory syncytial virus (see Chapters 4 and 10).
virus), whereas MVE virus is only found in Australia. Thus,
As indicated before, most human infections by JE virus
circulation of at least some of these viruses has occurred
do not result in invasion of the nervous system and encepha-
over widespread areas, as is the case for the dengue viruses
litis does not occur. Two laboratory experiments are of inter-
described in the preceding section. For most of the viruses
est in this regard. In one experiment, JE virus variants were
in this lineage, birds form the major vertebrate reservoir and
selected that failed to bind to mouse brain membrane receptor
culicine mosquitoes are the major vectors.
preparations. These mutants were attenuated for neuroinva-
JE virus is distributed throughout Asia, including Japan,
siveness and neurovirulence because their receptor-binding
India, Southeast Asia, Indonesia, the Philippines, and
preferences were altered. In a second experiment, passage
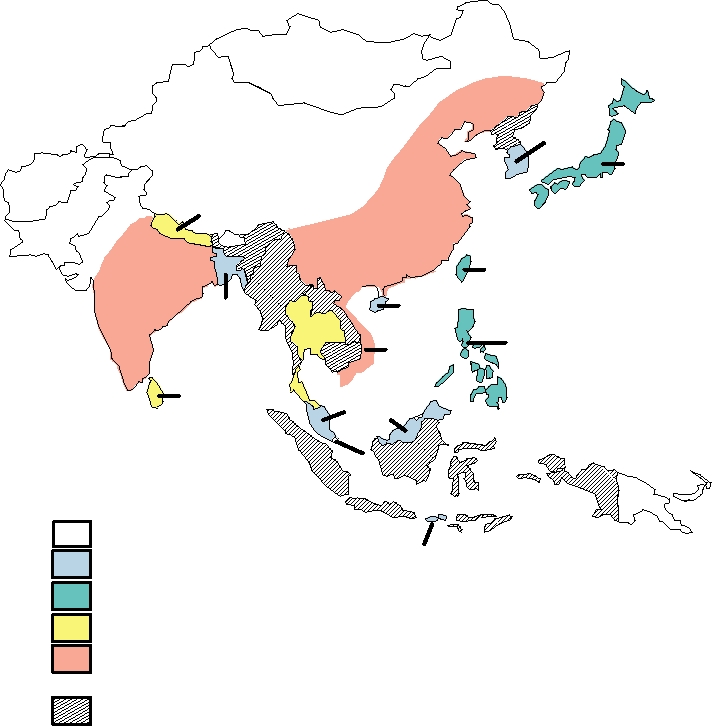
Borneo (Fig. 3.33). Reported cases of JE encephalitis aver-
of JE virus and of MVE virus in cultured cells selected for
age 30,00050,000 per year with 10,000 deaths, but the dis-
variants that bound to glycosaminoglycans (GAG). These
SouthKorea
(6)
Japan
(122)
Nepal
(1283)
China
(122,955)
Taiwan
India
(127)
(25,856)
Hong Kong
Bangladesh
(4)
Thailand
(5)
Philippines
(836)
Vietnam
(174)
(9745)
Sri Lanka
Malaysia
(1965)
(104)
Singapore
(10)
Reported Cases 19861990
None
Bali
120
(5)
21200
2012000
>2001
No case data, but endemism suspected
FIGURE 3.33 Range and reported cases of Japanese encephalitis, 19861990. Adapted from MMWR (1993) Vol. 42,
RR-1, p. 2. Since this detailed report, the first human cases were reported in Papua New Guinea in 1997, there were two fatal
cases on islands in the Torres Strait in 1995, and the virus was detected in mainland Australia (the Cape York Peninsula) in
1998.
variants were rapidly removed from the bloodstream when
But with the recent occurrence of outbreaks of encephalitis
inoculated into mice and were attenuated. It was suggested
in Europe, the Middle East, and North America the situa-
that GAGs present on cells and extracellular matrices result
tion changed. Not only has infection by the virus resulted in
in the removal of these variants from blood and tissues before
large numbers of cases of neurological disease in humans,
replication and neural invasion can take place. Evidence was
but domestic animals, especially horses, and wildlife, par-
presented that the attenuation of the live JE virus used as a
ticularly birds, have been severely impacted. The effect of
vaccine in China, called SA14-14-2, may have resulted, at
the virus in North America has been especially dramatic, as
least in part, from such an effect.
described in more detail in Chapter 8. Since its arrival in
1999, more than 20,000 Americans have become ill from
West Nile infection and more than 800 have died. The virus
West Nile Virus
has also had severe effects upon horses and many species
West Nile (WN) virus was first isolated in 1937 from the
of birds.
blood of an infected woman in the West Nile province of
Two lineages of WN virus are recognized. The lineage
Uganda. Until 1999 it was not considered an important path-
present in North America, Europe, the Middle East, India,
ogen, causing only sporadic cases of encephalitis in parts of
Australia (where the subtype present has been called Kunjin
Africa, Asia, and Europe and having little effect on wildlife.
virus), and parts of Africa, called lineage 1, contains both
virulent and attenuated strains and is responsible for WN
of contacts. Alligators were accidentally infected by feeding
disease. Lineage 2 is present only in sub-Saharan Africa and
them infected birds. In humans, transmission of the virus by
Madagascar and is mostly maintained in an enzootic cycle.
blood transfusion or via breast milk has occurred.
The association of the virus with significant outbreaks of dis-
Pathology of West Nile Disease
ease in humans, domestic animals, and birds, and the wide-
spread dispersion of the virus in Eurasia and the Americas,
About 80% of human infections appear to be asymptom-
appears to be the result of the emergence of a more virulent
atic. In the 20% of infections that result in clinical disease,
strain of the virus.
most result in a self-limited illness characterized by fever,
Various species of culicine mosquitoes are the princi-
headache, fatigue, malaise, muscle pain, and weakness. In
pal vectors of WN virus, although the virus has also been
fewer than 1% of infected humans does the virus cross the
isolated from species of Aedes, Coquillettidia, Culiseta,
bloodbrain barrier and cause neurological disease such
and Ochlerotatus, among others. In Europe and Africa the
as meningitis, encephalitis, or paralysis. In about 13% of
principal vectors are Culex pipiens, Cx. univittatus, and Cx
patients experiencing neurological disease, infection of
antennatus, in India Cx. vishnui, in Australia Cx. annuliros-
anterior horn cells of spinal motor neurons causes an acute
tris, and in North America Cx. pipiens, Cx. quinquefascia-
flaccid paralysis very similar to poliomyelitis resulting from
tus, and Cx. tarsalis, among others. During epidemics, from
poliovirus infection. The fatality rate for neuroinvasive dis-
0.1% to as many as 15% of Culex mosquitoes were found to
ease is about 10%, and many survivors, especially those with
be infected.
poliomyelitis type disease, never fully recover.
WN virus infects a wide spectrum of vertebrates. The res-
Infection with WN virus may be serious because the
ervoir of the virus is various species of birds. More than 150
virus interferes with the innate immune system (see Chapter
species of birds were shown to be infected by WN virus and
10). Several of the nonstructural proteins of the virus inter-
only in birds, with few exceptions, does the virus produce
fere with phosphorylation of the Janus kinases JAK1 and
high enough viremia titers to infect mosquitoes. Laboratory
Tyk2. This prevents the activation of the transcription fac-
studies showed that viremia titers of 105 to 107 are required to
tors STAT1 and STAT2 and their transport to the nucleus.
infect mosquitoes; at the lower titer fewer than 15% of feed-
Activation of these factors is required for the cell to respond
ing mosquitoes become infected, whereas at the higher titer
to the signaling of the interferons, normally a first line of
more than 70% become infected. Mammals in general do
defense against viral infection.
not generate such high viremia titers after infection by WN
Vaccines to protect horses have been developed. One is
virus. The maximum viremic titer in humans and horses, for
an inactivated virus vaccine. The other is a recombinant vac-
example, appears to be about 103. Among birds, grackles,
cine that uses canarypox virus to express WN virus antigens.
corvids (crows, ravens, jays, magpies), house finches, house
Two viruses for human use are also being developed. The
sparrows, shorebirds, hawks, and owls are most susceptible
first is an inactivated virus vaccine. The second is a chimeric
to the virus and show high mortality rates upon infection
YFWN live virus vaccine in which the prM and E proteins
(25100% in various studies). They develop sufficiently
of the 17D YF virus vaccine strain have been replaced with
high viremia to efficiently infect mosquitoes that feed upon
those of WN virus (see Chapter 11).
them. WN virus has also been isolated from amphibians and
reptiles, and the lake frog of Russia develops sufficiently
Other Flaviviruses of the JE Complex
high viremia that it might serve as a reservoir.
There are reports that WN virus can persist in infected
MVE virus is an Australian virus that is closely related to
animals for a considerable time. Virus could be isolated from
JE virus. It causes encephalitis in humans, but the number
experimentally infected ducks and pigeons for more than 3
of cases is small. Birds are the primary vertebrate reservoir,
months, for example. Such persistence could be important
and epidemics of MVE have been associated with wet years
in the persistence of the virus in nature. Also important is
when the mosquito population expands and nomadic water-
transovarial transmission, which occurs in many flavivi-
fowl invade regions that are normally too dry to support
ruses, and West Nile is no exception. During transovarial
them. Culex annulirostris is the primary vector for MVE.
transmission, the eggs laid by the mosquito are infected and
SLE virus is a North American virus belonging to the JE
the emergent mosquito is thus infected. This mechanism is
complex that causes regular epidemics of encephalitis in the
especially important in temperate climates where adult mos-
United States. The virus is widely distributed and cases of
quitoes die off during the winter and the species persists as
SLE encephalitis have been recorded in every state, with the
diapausing embryos or larvae.
majority of cases occurring in the Mississippi River valley,
WN virus can also be spread by means other than by mos-
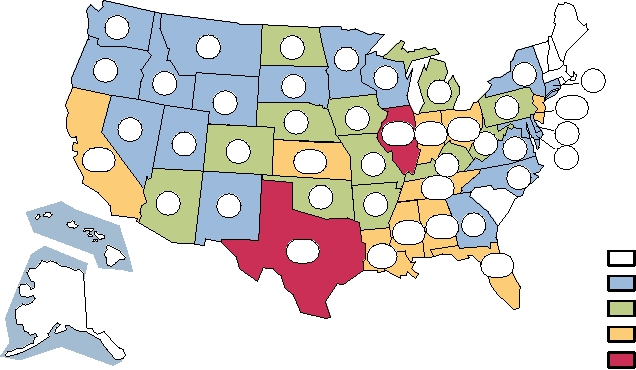
Texas, California, and Florida. Data for the years 19642003
quitoes, although the importance of such spread in the main-
are shown in Fig. 3.34. In the epidemic year 1975 there were
tenance and spread of the virus is unknown. Birds excrete
1815 cases of SLE encephalitis officially reported in the
virus in their feces, which can serve as a source of infection
United States, but in nonepidemic years there may be fewer
3
2
19
8
2
10
8
1
3
4
29
1
37
131
25
14
540 368 440
3
1
11
1
7
87
9
118
125
75
67
3
141
11
Cumulative Cases of SLE
34
94
9
1964 to 2003
336 149 5
978
116
0
365
1-10
11-100
101-500
>500
FIGURE 3.34 Distribution of cases of St. Louis encephalitis occurring between 1964 and 2003 in the United States,
shown by state. The large number of cases in Florida includes the most recent U.S. epidemic, which occurred in 1990,
during which Florida reported 223 cases and 11 deaths. Data came from Fields et al. (1996) p. 981, and MMWR, Summary
of Notifiable Diseases, 1996, 1997, 1998, 1999, 2000, 2001, 2002, 2003. St. Louis encephalitis was not a notifiable disease
nationwide until 1998. Recent reported cases were 24 in 1998, 4 in Florida in 1999, 2 in Texas in 2000, 79 in 2001 of which
71 were in Louisiana, 28 in 2002, and 41 in 2003. These have been incorporated into the state totals shown.
that 50 cases. The most recent epidemic occurred in 1990
other TBE viruses, can also be contracted by drinking raw
in Florida with 223 cases and 11 deaths. The case fatality
goat's milk and possibly other forms of raw milk. The virus
rate is about 7% overall, but is higher in the elderly. Most
has a tendency to set up persistent infection in experimental
infections by SLE are inapparent, as is the case for many
animals and possibly in humans as well. Although Ixodes
encephalitis viruses. The ratio of inapparent to clinical infec-
ticks are the primary vector, Dermacentor ticks and ticks of
tion is age dependent and varies from 800 to 1 in children
other genera are also capable of transmitting the virus. The
to 85 to 1 in the elderly. The virus is transmitted by Culex
distributions of two species of Ixodes ticks that are important
mosquitoes, and the primary vertebrate reservoirs are wild
vectors of TBE are shown in Fig. 3.35 together with the geo-
birds.
graphic range of endemic TBE disease.
Powassan virus is a member of the complex found in
North America and in Russia. In North America, 20 cases of
Tick-Borne Encephalitis Viruses
Powassan encephalitis have been reported since 1958.
The tick-borne encephalitis (TBE) viruses are impor-
All known TBE viruses cause encephalitis in humans with
tant pathogens in Europe and Asia, and there is also a rep-
the exception of Omsk hemorrhagic fever virus, which causes
resentative in North America. The viruses include Central
hemorrhagic fever in humans, as its name implies, in the
European encephalitis (CEE), louping ill, Russian spring-
absence of encephalitis. Two other members of this complex,
summer encephalitis (RSSE), Kyasanur Forest disease,
Kyasanur Forest disease virus and Alkhurma virus, which are
Omsk hemorrhagic fever, and Powassan viruses. Members
closely related and may represent isolates of the same virus,
of the TBE complex form a distinct group within the fla-
also cause hemorrhagic fever in humans but it is associated
viviruses (Fig. 3.28), but share 40% amino acid sequence
with encephalitis. Omsk hemorrhagic fever virus also dif-
identity with the mosquito-borne flaviviruses, showing their
fers from other TBE viruses in that its principal tick vector is
close relationship to other flaviviruses. Most TBE viruses are
Dermacentor reticulates rather than an Ixodes tick.
transmitted by Ixodes ticks and can cause a fatal encephali-
tis in humans. An inactivated virus vaccine is widely used
Cell Fusing Agent
in Central Europe to protect people exposed to ticks. Even
so, several thousand cases of TBE encephalitis occur each
A flavivirus called cell fusing agent was discovered in
year. The case fatality rate is 12%, with 1020% of survi-
laboratory cultures of Ae. aegypti cells in 1975. The relation-
vors having sequelae in the RSSE form. RSSE, and perhaps
ship of this virus to other flaviviruses is shown in Fig. 3.28.
Both ticks
Ixodes ricinus
Ixodes persulcatus
Endemic tick-borne encephalitis
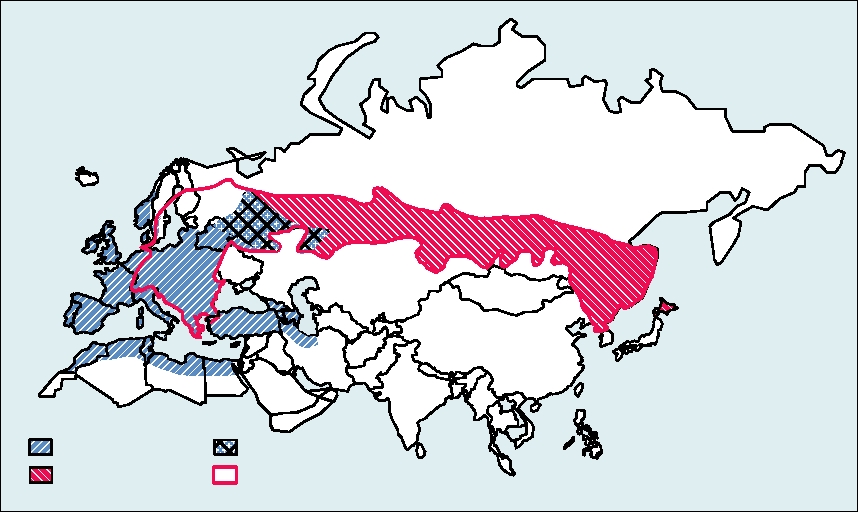
FIGURE 3.35 Geographic distribution of two major tick vectors of tick-borne encephalitis. Also shown is the major
region in which TBE is endemic. Adapted from Porterfield (1995) p. 207.
This virus is an insect only virus and is not known to infect
is the presence of two proteases rather than one. The NS3
vertebrates. Very recently, new isolates of a strain of cell
protease is shared with the flaviviruses but requires NS4A
fusing agent have been made from wild-caught mosquitoes
as a cofactor rather than NS2B. The second protease, which
in Puerto Rico belonging to at least two genera, Aedes and
has a catalytic cysteine, is present in NS2 and its only known
Culex. Remarkably, DNA sequences related to cell fusing
cleavage function in viral replication is to cleave the NS2
agent have been identified in the genomes of wild-caught
NS3 bond.
A second difference is the lack of a 5′ cap and the pos-
mosquitoes, presumably having integrated into the mos-
quito genome at some time in the distant past. This and other
session instead of an IRES, so that initiation of translation
recent isolates of new flaviviruses has led to the suggestion
of the plus-strand genome is not cap-dependent but uses an
that there are as yet many flaviviruses in nature that remain
IRES as does poliovirus. A third difference is the production
to be identified.
of a small (17 kDa), short-lived protein called F (for frame
shift) or ARFP (for alternative reading frame protein) that
is encoded within the C protein gene in a different reading
Genus Hepacivirus
frame. Translation of this protein requires initiation at the 5′
Hepatitis C virus (HCV) forms a second genus in the
end of the polyprotein followed by a frameshift near residue
Flaviviridae. The virus was discovered in 1989 as a causa-
11 of the capsid protein. There is evidence that it is produced
tive agent of nonA-nonB hepatitis in humans. Despite the
in infected humans but it is not known if this protein plays a
inability to grow the virus in culture or in a small animal
role in virus replication. Of note is the fact that no other plus-
model, the complete genome sequence of the virus was estab-
strand RNA virus is known to produce two different proteins
lished using the methods of modern biotechnology and veri-
from two different reading frames in the same nucleotide
fied by injection of viral RNA produced from cDNA clones
sequence, although this phenomenon occurs in several other
into the liver of a chimpanzee, the only animal other than
classes of viruses.
humans that is infectible by the virus. The HCV genome,
HCV also differs in the way that RNA replication is
which is slightly smaller than those of the flaviviruses and
anchored to a membrane. RNA replication in plus-strand
pestiviruses, has an organization similar to those of the other
RNA viruses occurs in association with membranes. In fla-
members of the family (Fig. 3.27). It has a number of impor-
viviruses, the RNA polymerase is thought to associate with
tant differences from the genome of members of the genus
membranes by means of its association with membrane
Flavivirus, some of which are illustrated in the figure. One
bound proteins such as NS4A or NS4B. In HCV, the RNA
polymerase NS5B is itself anchored in the membrane by a
allow a complete virus replication cycle with the release of
C-terminal transmembrane anchor. Interestingly, this anchor
infectious virus will allow more rapid progress in the future.
is required for RNA replication and the HCV sequence can-
A second advance has been the use of immunodeficient mice
not be substituted with that from the pestivirus bovine viral
(severe combined immunodeficiency or SCID mice) into
diarrhea virus. Thus, this anchor plays a role in RNA rep-
which have been grafted human liver cells. These can be
lication other than simply anchoring the polymerase in the
infected by HCV and although the numbers of such animals
membrane.
is limiting, they possess obvious advantages over the use of
Another interesting difference is the cleavage of the N-
chimps.
terminal capsid protein from the polyprotein precursor. The
capsid protein is anchored in the membrane by a C-terminal
Natural History of HCV
transmembrane anchor, as described earlier for flaviviruses.
In flaviviruses the capsid protein is cleaved from this sig-
HCV is a causative agent of blood-borne hepatitis in man.
nal sequence anchor by the NS3 protease, but in HCV it is
In the United States, HCV was once spread primarily through
cleaved by a cellular protein, signal peptide peptidase.
transfusion of contaminated blood, but the development of
Because of its importance as a human disease agent,
a diagnostic screen for the virus has virtually eliminated
HCV has been the subject of intensive study. Progress has
this source of infection in the developed world. However,
been relatively slow because the only animal model for the
the virus continues to be transmitted through the sharing
disease is the chimpanzee, which are rare and expensive,
of needles by drug users. The virus can also be transmitted
limiting the number of experiments that can be performed,
sexually or from mother to child but these mechanisms are
and because until very recently there was no cell culture sys-
inefficient. There are additional mechanisms of transmission
tem in which the virus would undergo a complete replication
that are not well understood. In some developing countries,
cycle and release infectious virus. Studies in cultured cells
circumcision or scarification practices may be important in
have relied upon the expression of parts of the genome in
the spread of the virus.
expression vectors, and more recently upon the replication of
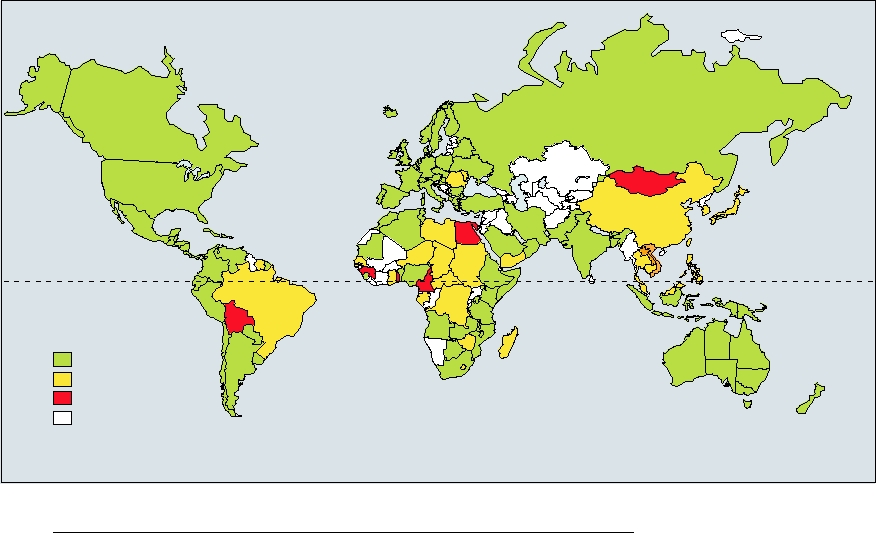
HCV is worldwide in distribution, as illustrated in
Fig. 3.36. It has been estimated that ∼3% of the world's
truncated versions of the genome called replicons. Replicons
encode all of the genes required for RNA replication but lack
population, 170 million people, are infected by the virus.
the genes encoding structural proteins. Thus, only part of
The highest infection rate found was among Egyptian blood
the virus life cycle can be studied using these reagents. The
donors, where up to 19% were seropositive for HCV, which
very recent development of systems using cultured cells that
may have resulted in part from past treatment for bilharziasis
Equator
Prevalence
(% of population infected)
1 to 2.4
2.5 to 10
>10
No data
FIGURE 3.36 Worldwide prevalence of hepatitis C as of April 2003 based upon published data. Map was found at:
http://www.reliefweb.int/rw/RWB.NSF/db900LargeMaps/SKAR-64GDV4?OpenDocument.
using inadequately sterilized needles. There are six differ-
RNA polymerase NS5B and inhibit its activity, two inhibi-
ent clades or genotypes of the virus, which differ by more
tors of the viral NS34A protease, and three compounds that
than 30% in nucleotide sequence and are numbered from 1
modulate the immune system. Other drugs are also being
to 6. In turn, each clade has many isolates that may differ by
studied as possible antivirals.
up to 25% in nucleotide sequence, so that the clades can be
subdivided into subclades called a, b, etc. These different
HCV Suppression of the Immune Response
viruses all cause the same disease but differ in the severity
of the disease caused and in their ease of cure. Genotype 1
In order to establish a chronic infection, HCV interferes
is the clade commonly found in the United States and prob-
with many aspects of both the innate and adaptive immune
ably became widespread only with the introduction of blood
responses of the host. The importance of such interference
transfusion in the 1940s. In Africa and Asia, the virus has
for chronicity and the persistence of the virus in nature is
been endemic for a long time, and the different clades have
illustrated by the fact that the virus interferes in so many dif-
different geographic distributions. Thus, for example, clade
ferent ways. The immune system is described in some detail
5 is commonly found only in South Africa, clade 4 is widely
in Chapter 10. Here we note that the first line of defense
distributed in the Middle East, clade 6 in eastern Asia, and
against viral infection is the production of type 1 interferons
(IFN) α and β, components of the innate immune system.
clade 2b in the Mediterranean and the Far East.
The NS34A protease of HCV interferes with the induction
of IFN by cleaving two intermediates, called MAV5 and
HCV Disease and Its Treatment
TRIF, in two different but overlapping activation pathways.
Infection with HCV can be extremely serious. The ini-
MAV5 is required in the pathway that starts from an intra-
tial infection may cause no disease or may result in hepatitis
cellular sensor of double-strand RNA called RIG-1, whereas
accompanied by jaundice, but fulminant liver failure is rare.
TRIF is required in the pathway that starts from a membrane
However, in 7080% of infections, the infection becomes
bound sensor of double-strand RNA called Toll-like recep-
chronic. During chronic infection, up to 1012 viruses are
tor 3 (see Chapter 10). The result is that both pathways are
produced each day and turn over with a half-life of about 3
disabled.
hours, and the more or less constant viral load in the blood
The HCV core protein interferes with the activity of any
is 103107 per ml. This chronic infection is well tolerated
IFN that might be produced. It induces the expression of cel-
by some and in a minority of cases spontaneous remission
lular proteins called SOCS1 and SOCS3. These block the
may occur in the absence of medical intervention. However,
JAKSTAT pathway by which IFN induces the production
in many persons chronic hepatitis results. Most seriously,
of hundreds of proteins required for defense against viral
in about 20% of chronic infections liver cirrhosis develops
infection (Chapter 10). Protein NS5A independently inter-
after a long lag, usually more than 20 years, and hepato-
feres with the IFN system in at least two ways. It induces
cellular carcinoma develops in up to 2.5%. Liver failure due
the production of IL-8, which attenuates the expression of
to HCV infection is the leading cause of liver transplantation
genes induced by the activity of IFN. It also binds to a pro-
in the United States.
tein called PKR that is induced by IFN, thereby inhibiting its
The current treatment for chronic HCV infection is injec-
activity. Protein E2 also inhibits PKR. Other HCV proteins
tion of interferon-α conjugated to polyethylene glycol,
are also known to interfere with the activity of IFN.
which increases its stability, together with the inhibitor riba-
HCV also interferes with the adaptive immune system.
virin. This treatment results in curing the infection in about
Interestingly, instead of a general interference with the adap-
half the cases but the cure rate depends upon the genotype of
tive system, as happens with HIV, for example, that cripples
the virus. In one trial, 42% of patients chronically infected
immune responses against all pathogens, the modulation
with genotype 1 HCV were cured whereas patients chroni-
by HCV is limited to HCV-specific responses, leaving the
cally infected with genotype 2 or 3 virus exhibited a cure
immune system free to control other viral infections. The
rate of 80%. This treatment is not only expensive but rela-
mechanisms by which this occurs are incompletely under-
tively toxic and many patients tolerate it poorly. This con-
stood. What is known is that successful clearance of HCV
sideration, as well as the fact that half the patients show no
infection in humans is associated with a strong T-cell
response, both CD8+ and CD4+, and that immunologic mem-
effect or only transient relief from this treatment, has led
to intense efforts to develop new treatments. These include
ory results such that although reinfection by HCV can occur,
efforts to develop vaccines as well as efforts to develop anti-
it does not lead to chronic infection. In humans in which the
infection becomes chronic, CD8+ cytotoxic T cells are rela-
viral agents that will interrupt virus replication or prevent
the virus from interfering with the host defenses against the
tively few and these T cells recognize fewer epitopes. In one
study, CD4+ helper T cells from persistent infections recog-
virus. Antivirals currently in clinical trials include nucleo-
side analogues that when incorporated into viral RNA result
nized very few epitopes whereas those from humans who
in chain termination, two compounds that bind to the viral
cleared the infection recognized up to 14 different epitopes.
HCV-Related Viruses
sheep (BDV). These three viruses share more than 70%
amino acid sequence identity and exhibit extensive serologi-
Viruses related to HCV, called GB viruses (from the
cal cross-reactivity. Their genome organization is similar to
initials of a surgeon with hepatitis from which they were
those of other viruses in the family (Fig. 3.37). Pestiviruses
first isolated), are known. GBV-A and GBV-B viruses have
have also been isolated from a number of other mammals
a genome organization very similar to that of HCV, but
including giraffe, deer, bison, bongo, alpaca, and reindeer.
share little amino acid sequence identity with HCV or with
The taxonomic status of these isolates is still unclear. Some
each other. They may eventually be classified as new gen-
are classified as strains of one of the three viruses just listed
era within the Flaviviridae, more closely related to genus
but some, at least, may represent other species of pestivirus
Hepacivirus than to genus Flavivirus or genus Pestivirus.
BVDV exhibits an important and interesting disease
A third virus, GBV-C, also called hepatitis G virus or HGV,
syndrome in cattle. Animals infected as adults by the virus
is related to GBV-A. These three viruses appear to be widely
may exhibit no disease or may have symptoms that include
distributed and establish chronic infections in humans, but
diarrhea, but they recover uneventfully. However, when a
there is no evidence that they cause disease.
pregnant cow is infected by the virus, infection of the fetus
may cause the fetus to become immunologically tolerant to
the virus, resulting in a chronic infection that lasts for the life
Genus Pestivirus
of the animal. Such in utero infection may lead to develop-
Three closely related viruses belonging to the genus
mental abnormalities or runting in the calf, and may render
Pestivirus are important pathogens of domestic animals
the calf sensitive to infection by other microorganisms, all
and have been well characterized. These are bovine viral
of which have serious economic effects. A more interesting
diarrhea virus (BVDV), classical swine fever virus (CSFV)
effect of the chronic infection, however, is the development
(also called hog cholera virus), and border disease virus of
in some animals of fatal mucosal disease at the age of 12
Structural Proteins
Nonstructural proteins
A
noncytopathic (ncp) BVDV
NS3
NS2
(BVDV SD1)
Translation, processing
little cleavage, no NS3
B
cytopathic (cp) BVDV
Ub
NS2
NS3
(BVDV Osloss)
partial Ub cleavage produces NS3
C
cp BVDV
Ub
NS2
NS3
NS3
(BVDV CP1)
Ub cleavage produces NS3 from a
duplicated gene
D
Npro
Npro
cp BVDV
NS2
NS3
NS3
(BVDV 515CP)
Cleavage by Npro autoprotease produces
pro
N
NS3 from a duplicated gene
Cleavage Motif
Npro autoprotease (papain-like)
Nucleocapsid protein
Ub Ubiquitin
Virion glycoproteins
FIGURE 3.37 Genome organization of cytopathic and noncytopathic strains of the pestivirus BVDV. (A) In noncytopathic
(wild-type) strains little cleavage occurs between NS2 and NS3. In cytopathic strains, NS3 is produced either by an upstream
insertion of ubiquitin (see B), insertion of multiple ubiquitin sequences plus duplication of NS3 sequences (see C), or
duplication of the Npro proteinase and its insertion immediately upstream of a duplicated NS3 (see D). Data for this figure
came from Meyers and Thiel (1996).
Search WWH :