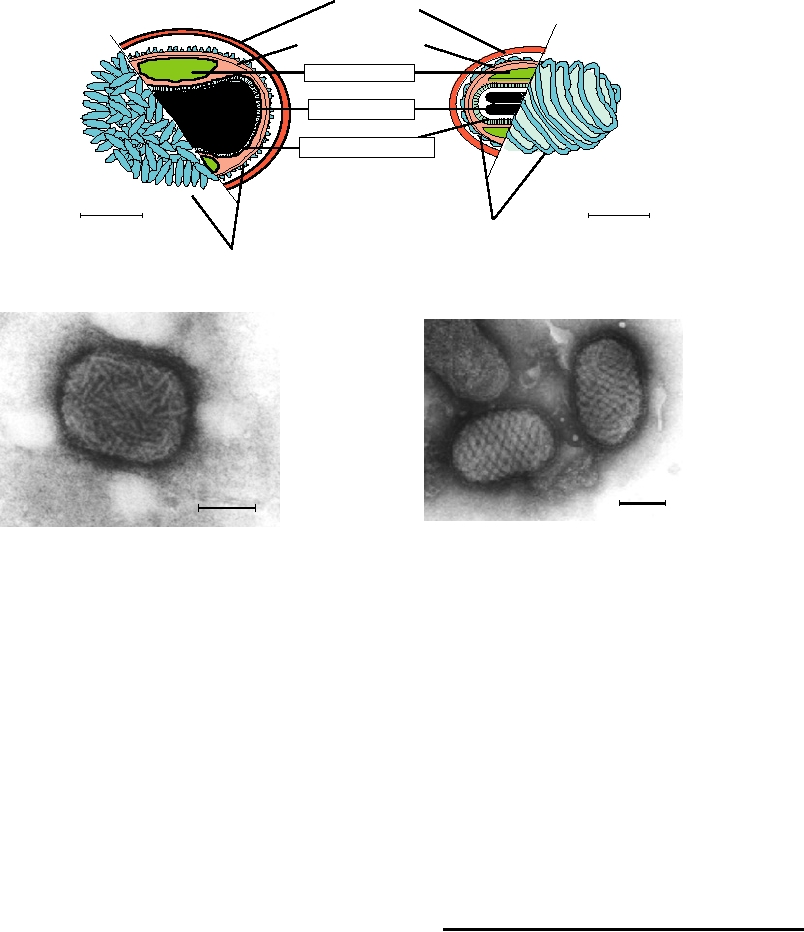
Envelope
A. Orthopox
B. Parapox
Surface membrane
(lipid-containing)
Lateral bodies
Nucleoprotein
Core "membrane"
100 nm
100 nm
Surface filament
Surface Tubule
C. Outer Surface Orthopox
D. Outer Surface Parapox
100 nm
100 nm
FIGURE 2.24
Morphology of orthopox and parapox virions. (A) and (B) Diagrams of orthopox and parapox virions.
At the far left and right are shown the surfaces of the particles as they are isolated from infected cells, with the outer
tubules or the outer filament shown in turquoise. The inner parts of each diagram show the enveloped particle in cross
section illustrating the core membrane, the lateral bodies, and the nucleoprotein. Adapted from Fenner and Nakano
(1988). (C) Purified vaccinia virus negatively stained with phosphotungstate; (D) Image of the outer surface of Orf
parapox virus. Images in (C) and (D) were kindly provided by Prof. Stewart McNulty, Veterinary Sciences Division,
Queens University of Belfast.
Parapox virions are similar to orthopox virions. However,
Vaccinia Virus
their morphology is detectably different, as illustrated in
The poxviruses, large DNA-containing viruses, also have
Fig. 2.24.
lipid envelopes. In fact, they may have two lipid-contain-
ing envelopes. The structures of poxviruses belonging to two
different genera, Orthopox and Parapox, are illustrated in
ASSEMBLY OF VIRIONS
Fig. 2.24. Electron micrographs of the orthopox virus vac-
cinia virus and of a parapox virus are also shown.
Self Assembly
Vaccinia virus has been described as brick shaped. The
Virions self-assemble within the infected cell. In most
interior of the virion consists of a nucleoprotein core and
cases, assembly appears to begin with the interaction of one
two (in vertebrate viruses) proteinaceous lateral bodies.
or more of the structural proteins with an encapsidation sig-
Surrounding these is a lipid-containing surface membrane,
nal in the viral genome, which ensures that viral genomes
outside of which are several virus-encoded proteins present
are preferentially packaged. After initiation, encapsidation
in structures referred to as tubules. This particle is called
continues by recruitment of additional structural protein
an intracellular infectious virion. As its name implies, it is
molecules until the complete helical or icosahedral structure
present inside an infected cell, and if freed from the cell it is
has been assembled. Thus, packaging of the viral genome is
infectious. A second form of the virion is found outside the
coincident with assembly of the virion, or of the nucleocap-
cell and is called an extracellular enveloped virion. This sec-
sid in the case of enveloped viruses. The requirement for a
ond form has a second, external lipid-containing envelope
packaging signal may not be absolute. In many viruses that
with which is associated five additional vaccinia proteins.
contain an encapsidation signal, RNAs or DNAs lacking
This form of the virion is also infectious.
such a signal may be encapsidated, but with much lower effi-
of retroviruses, some of which assemble a nucleocapsid
ciency. For some viruses, there is no evidence for an encap-
during virus budding, was discussed earlier. In these viruses,
sidation signal.
morphogenesis is a coordinated event.
Assembly of the TMV rod (Fig. 2.2) has been well stud-
The forces that result in virus budding are not well under-
ied. Several coat protein molecules, perhaps in the form of
stood for most enveloped viruses. In the case of the alphavi-
a disk, bind to a specific nucleation site within TMV RNA
ruses, there is evidence for specific interactions between
to initiate encapsidation. Once the nucleation event occurs,
the cytoplasmic domains of the glycoproteins and binding
additional protein subunits are recruited into the structure
sites on the nucleocapsid proteins. The model for budding of
and assembly proceeds in both directions until the RNA is
these viruses is that the nucleocapsid first binds to one or a
completely encapsidated. The length of the virion is thus
few glycoprotein heterodimers at the plasma membrane. By
determined by the size of the RNA.
a process of lateral diffusion, additional glycoprotein het-
The assembly of the icosahedral turnip crinkle virion has
erodimers move in and are bound until a full complement
also been well studied. Assembly of this T=3 structure is
is achieved and the virus is now outside the cell. Additional
initiated by formation of a stable complex that consists of
free energy for budding is furnished by lateral interactions
six capsid protein molecules bound to a specific encapsida-
˙˙tween the glycoproteins, which form a contiguous layer
tion signal in the viral RNA. Additional capsid protein dim-
on the surface (Fig. 2.18C). This model accounts for the
ers are then recruited into the complex until the structure is
regularity of the virion, the one-to-one ratio of the structural
complete.
proteins in the virion, and the requirement of the virus for its
It is probable that most other viruses assemble in a man-
own glycoproteins in order to bud.
ner similar to these two well-studied examples. At least
In other enveloped viruses, however, there is little
some viruses deviate from this model, however, and assem-
evidence for nucleocapsidglycoprotein interactions. The
ble an empty particle into which the viral genome is later
protein composition of the virion is usually not fixed, but
recruited. It is also known that many viruses will assem-
can vary within limits. In fact, glycoproteins from unre-
ble empty particles if the structural proteins are expressed
lated viruses can often be substituted. In the extreme case
in large amounts in the absence of viral genomes, even if
of retroviruses, noninfectious virus particles will form that
assembly is normally coincident with encapsidation of the
are completely devoid of glycoprotein. The matrix proteins
viral genome in infected cells.
appear to play a key role in the budding process, as do other
proteinprotein interactions that are yet to be determined.
Enveloped Viruses
Maturation Cleavages in Viral
The nucleocapsids of most enveloped viruses form within
Structural Proteins
the cell by pathways assumed to be similar to those described
above. They can often be isolated from infected cells, and for
For most animal viruses, there are one or more cleavages
many viruses the assembly of nucleocapsids does not require
in structural protein precursors during assembly of virions
viral budding or even the expression of viral surface glyco-
that are required to activate the infectivity of the virion.
proteins. After assembly, the nucleocapsids bud through a
Interestingly, these cleavages may either stabilize or destabi-
cellular membrane, which contains viral glycoproteins, to
lize the virion in the extracellular environment, depending on
acquire their envelope. Budding retroviruses were illustrated
the virus. Many of these cleavages are effected by viral pro-
in Fig. 2.21 and budding rhabdoviruses in Fig. 2.23. A gal-
teases, whereas others are performed by cellular proteases
lery of budding viruses belonging to other families is shown
present in subcellular organelles. Virions are formed by the
in Fig. 2.25. The membrane chosen for budding depends on
spontaneous assembly of components in the infected cell,
the virus and depends, in part if not entirely, on the mem-
sometimes with the aid of assembly factors ("scaffolds")
brane to which the viral glycoproteins are directed by signals
that do not form components of the mature virion. For most
within those glycoproteins. Many viruses bud through the
nonenveloped viruses, complete assembly occurs within the
cell plasma membrane (Figs. 2.25BF); in polarized cells,
cell cytoplasm or nucleoplasm. For enveloped viruses, final
only one side of the cell may be used. Other viruses, such as
assembly of the virus occurs by budding through a cellu-
the coronaviruses and the bunyaviruses, use the endoplasmic
lar membrane. In either case, the virion must subsequently
reticulum or other internal membranes. The herpesviruses
disassemble spontaneously on infection of a new cell. The
replicate in the nucleus and the nucleocapsid assembles in
cleavages that occur during assembly of the virus potentiate
the nucleus; in this case, the first budding event is through
penetration of a susceptible cell after binding of the virus
the nuclear membrane (Fig. 2.25A).
to it, and the subsequent disassembly of the virion on entry
Although the nucleocapsid of most enveloped viruses
into the cell. A few examples will be described that illus-
assembles independently within the cell and then buds to
trate the range of cleavage events that occur in different virus
acquire an envelope, exceptions are known. The example
families.
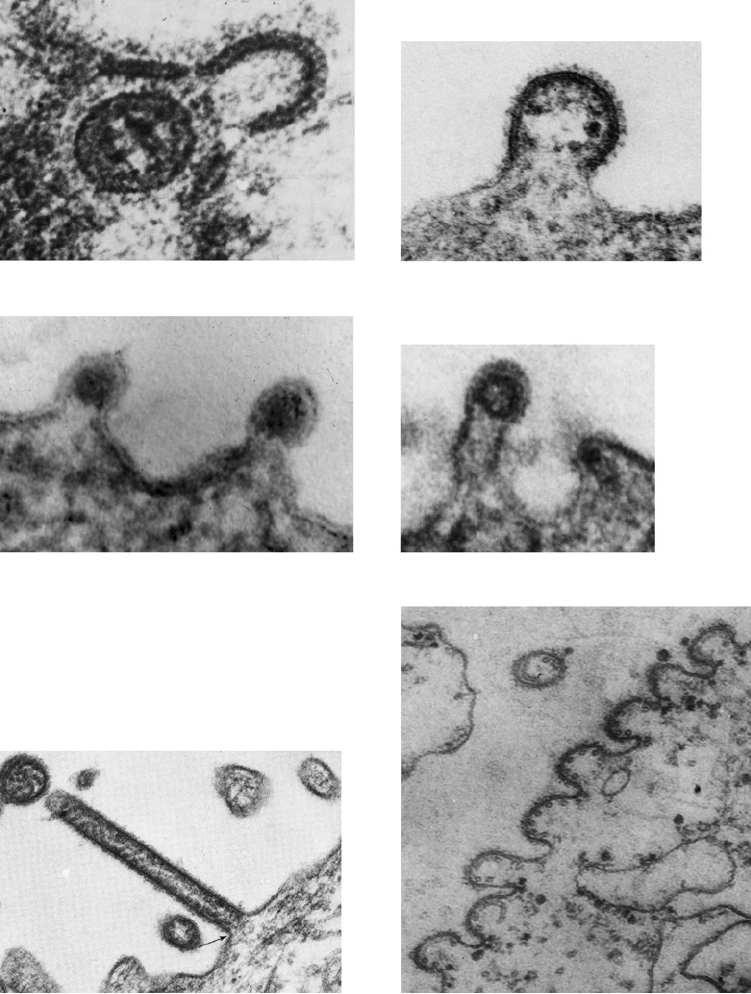
A. Herpes
B. Machupo
C. Sindbis
D. Rubella
F. SV5 Round Particle Bud
E. SV5 Filamentous Bud
FIGURE 2.25
Gallery of budding figures of viruses representing several different families. (A) Thin section of a
herpes simplex virion (Herpesviridae) in an infected Hep-2 cell. The particle is apparently coated with an inner envelope,
and is in the process of acquiring its outer envelope from the nuclear membrane. From Roizman (1969). (B) Machupo
virus (Arenaviridae) budding from a Raji cell. From Murphy et al. (1969). (Magnification: 120,000×). (C) Sindbis virus
(Togaviridae) budding from the plasma membrane of an infected chicken cell. From Strauss et al. (1995). (160,000×).
(D) Rubella virions (Togaviridae) budding from the surface of a BHK cell. From Higashi et al. (1976). (190,000×). (E) A
portion of the cell surface with SV5 filaments (Paramyxoviridae) in the process of budding. From Compans and Choppin
(1973). (45,000 ×). (F) A row of SV5 virions budding from the surface of a monkey kidney cell. Cross sections of the
nucleocapsid can be seen within several of the particles. From Compans et al. (1966). (87,000×).
Search WWH :